Academic Editor: Tao Wang
Background: Wound infection represents a frequent trouble following
open saphenous vein harvesting in cardiac surgery. Platelets’ growth factors are
crucial for the healing process. Prophylactic platelet rich plasma (PRP)
application on leg wound might reduce the incidence of saphenous vein harvest
site infections in patients undergoing coronary artery bypass graft surgery
(CABG). Methods: Between January 2009 and December 2020, 987 consecutive
patients underwent CABG using saphenous vein as conduit graft and were
retrospectively divided into two groups. All patients had standard surgical leg
wound closure and wound care, but treatment group received adjunctive topical
application of PRP (no-PRP and PRP group, respectively). The primary outcome was
wound infection. Results: Saphenous vein harvest site infection
rate was similar between PRP (3.5%) and No-PRP (5.2%) group, p = 0.215. The
ASEPSIS score was lower for the PRP group (PRP: 3.6
Coronary artery bypass grafting (CABG) surgery is the most appropriate revascularization strategy for patients with complex multivessel coronary artery disease and reduced left ventricular ejection fraction, improving prognosis and quality of life [1]. Despite the increased tendency of using arterial grafts considering their improved long term patency, the saphenous vein (SV) remain the most frequently used conduit in CABG especially in older patients [2]. The easy accessibility, possibility of simultaneous harvesting, and its length make the SV an almost-ideal conduit for CABG [2]. Unfortunately, SV grafts have two main limitations: high risk of graft failure (15%–20% within a year) and harvest-site complications [3, 4]. The reported incidence of the saphenous vein harvest site infection (SVHI) ranges between 1% and 24% [5, 6]. Although endoscopic SV graft harvesting reduces the rate of harvest-site complications, this technique has controversial impact on the graft patency [7, 8]. It has been reported that SV graft patency has been consistently lower with endoscopic harvesting than with open SV harvesting, probably because of mechanical factors during the procedure (e.g., overstretch injury) [8]. Therefore, some surgeons still prefer to perform a safe and gentle SV harvesting through a full open incision. Moreover, Diabetes Mellitus, that is associated with a 2 to 4-fold increased mortality risk from coronary artery disease, complicates the wound healing process in patient undergoing CABG. In this complex scenario, platelets growth factors play a pivotal role in tissue regeneration and healing. Autologous platelets are easily collected and concentrated by processing platelet-rich plasma (PRP). PRP contains at least 6 platelet growth factors, which have been associated with a beneficial wound healing [9, 10, 11, 12]. Recently, much research focused on the effectiveness and safety of PRP in management of wound infection. So far, due to the novelty of PRP, few studies were published on its efficacy in human subjects. In view of the limited clarity of available data, the aim of our study was to examine the effects of the topical PRP use in leg wounds after SV harvesting.
The purpose of this study was to determine if the incidence of perioperative saphenous vein harvest site infection could be effectively reduced by topical PRP application during the wound closure.
We performed a retrospective observational single center non-randomized cohort
study recruiting all patients undergoing cardiac surgery at the “Magna Graecia”
University, Catanzaro, Italy. Patients were considered eligible if they were
receiving an elective CABG using a saphenous vein graft with an open technique.
Exclusion criteria included lower leg surgical intervention, severe peripheral
vascular disease, emergent surgery, dialysis-dependent renal failure, chronic
steroid administration, previous CABG, or preoperative anemia (Hb
Primary outcome was the incidence of lower extremities surgical site infections. This was determined through assessment of the ASEPSIS score [13]. Only the moderate and severe complaints were included for incidence analysis. The wound evaluation was performed daily during the hospitalization and at 1-month follow-up. Data were retrospectively analysed. The need for informed consent was waived due to the retrospective study design.
Details of PRP preparation have been previously described [10]. Eighteen milliliters of whole blood were taken from the central line and processed to obtain a red-colored platelet gel, rich in growth factors released from the alpha granules in the activated platelets. About 8 mL of PRP was obtained out of 18 mL of blood. It was stored vertically at room temperature until its application. PRP was spread on the leg wound before the closure of subcutaneous tissue (Fig. 1). Care was taken not to remove any of the activated PRP with swabs during closure of the wound.
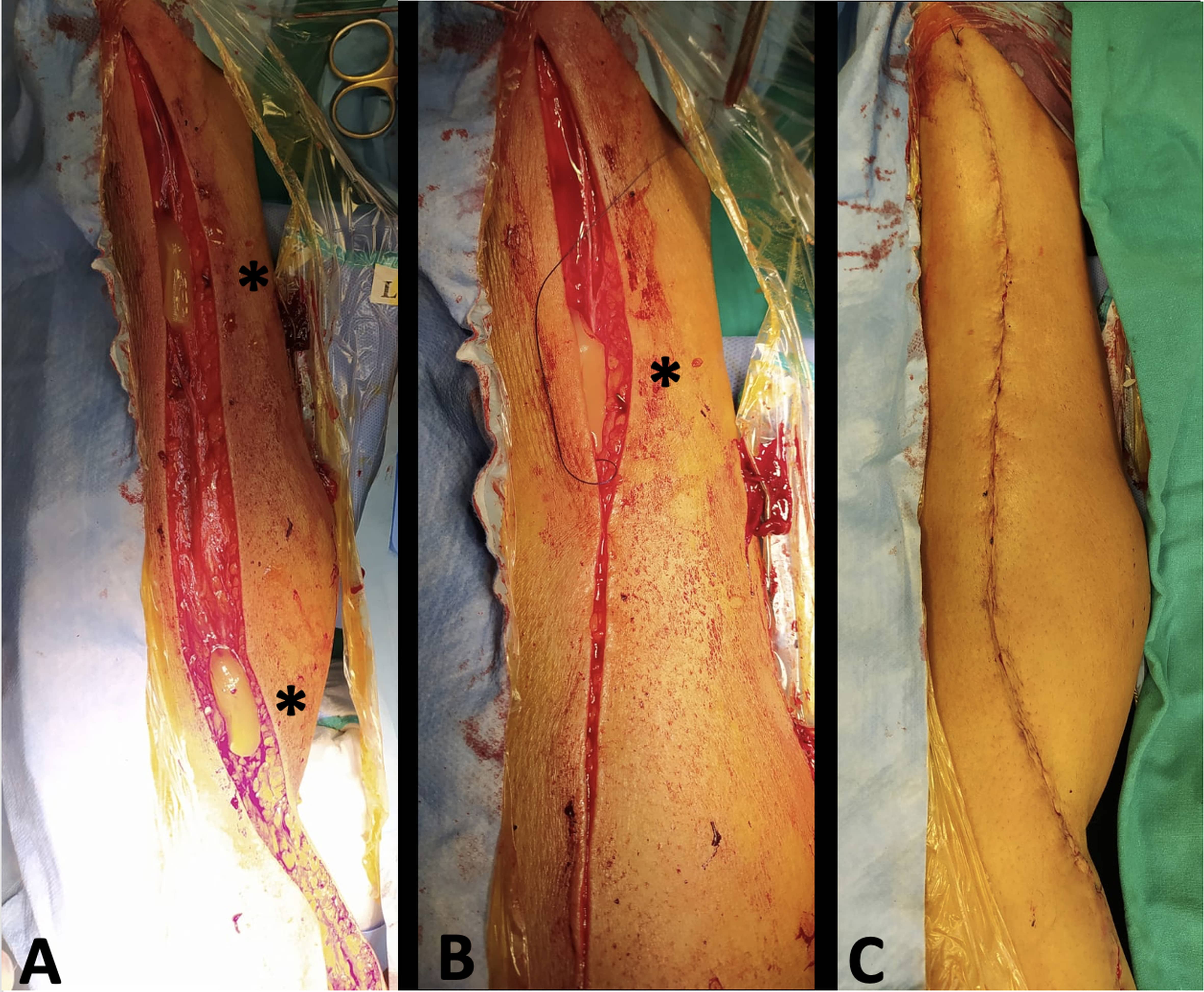 Fig. 1.
Fig. 1.Saphenous vein is harvested to be used for coronary artery bypass graft. (A) During leg closure, platelet rich plasma (“yellow gel” pointed by the *) is applied to enhance wound healing and prevent saphenous vein harvesting site infection. (B) The leg wound is closed keeping the platelet rich plasma (“yellow gel” pointed by the *) in the subcutaneous tissue. (C) The leg wound is closed.
Preoperative shaving and bath with povidone-iodine soap solution before surgery were performed for all patients. Considering saphenous vein harvesting, skin was incised with scalpel and electrocautery was never used. Full open technique was used in all patients. Leg closure was always achieved before heparin administration. The harvest wound was closed with intracutaneous poliglecaprone 3/0 according to our routine. In the treatment group, a plenty amount of PRP was spread on the wound immediately before closure. The leg was then wrapped with sterile elastic bandages. Cardiopulmonary bypass and surgical techniques were standardized and similar throughout the study period.
All patients received gradient compression stockings for 30 postoperative days. Wound dressing was performed daily. In case of signs of wound infections, microbiologic analysis was performed immediately. In both groups, antibiotic prophylaxis consisted of intraoperative administration of 2 g of Cefazolin. Post-operatively, antibiotic prophylaxis continued with administration of cefazolin 2 g twice a day, unless dose adjustment were required for impaired kidney function. In the case positive cultures or clinical signs of infection, the antibiotic treatment was modified according to antibiogram. After discharge, patients were admitted in a physical rehabilitation center for at least 15 days. Professional nurses took care of the wounds. Any complications in wound healing were reported to our department for readmission of the patients. After, patients were visited for a 30-day follow-up at our outpatient clinic, where wounds were evaluated by a cardiac surgeon.
Statistical analysis was performed by the SPSS program for Mac, version 25.0
(IBM SPSS Statistics for Macintosh., Armonk, NY). Continuous variables are
presented as mean
A total of 953 consecutive patients underwent CABG using the great saphenous
vein as conduit were considered eligible. Patients submitted to saphenous vein
harvesting with bridging or endoscopic technique (235) in the same study period
were not included into the study. Thirty-four patients were excluded because of
previous CABG (10), lower leg surgical intervention (2), severe peripheral
vascular disease (9), dialysis-dependent renal failure (9). Autologous PRP was
not obtained in 4 patients because of primary or drugs related severe clotting
abnormalities. PRP was spread on venous harvesting site before the closure of
subcutaneous tissue of 452 patients. The control group of 501 patients (No PRP
group) received a standard closure of the great saphenous vein harvest site. No
treatment-related adverse events were observed. Every patient was submitted to
CABG, however, 136 patients (30.1%) of the PRP group and 161 (32.1%) cases of
the control group underwent to combined procedures (CABG and surgery of the
aortic valve, CABG and surgery of the mitral valve, CABG and surgery for left
ventricular aneurysm, CABG and surgery for aortic aneurysm) (p = 0.173). The
groups were homogeneous for preoperative risk factors considering both the
overall cohort and the subgroup of diabetic patients (Table 1). Mean length of
the saphenectomy incisions was 43.8
| Overall population | PRP (n = 452) | No PRP (n = 501) | p value |
| Age (years) (median; IQR) | 67 (13) | 67 (14) | 0.588 |
| Male gender (%, n) | 77.9% (352) | 76.0% (381) | 0.504 |
| BMI (kg/m |
27.51 |
27.14 |
0.211 |
| COPD (%, n) | 15.2% (69) | 13% (65) | 0.310 |
| Diabetes Mellitus (%, n) | 41.8% (189) | 38.9% (195) | 0.363 |
| Diabetes Mellitus Type 2 | 164 (87%) | 169 (86%) | 0.976 |
| Smoking (%, n) | 35.6% (161) | 30.53% (153) | 0.096 |
| Hypertension (%, n) | 35.3% (160) | 33.3% (167) | 0.503 |
| Dyslipidemia (%, n) | 30.9% (140) | 27.1% (136) | 0.193 |
| Creatinine (mg/dL) (mean |
1.02 |
1.01 |
0.467 |
| Among patients with DM | DM with PRP (n = 189) | DM without PRP (n = 195) | p value |
| Age (years) (median; IQR) | 67 (12) | 68 (12) | 0.873 |
| Male gender (%, n) | 73% (138) | 70.2% (137) | 0.549 |
| BMI (kg/m |
27.62 |
26.83 |
0.096 |
| COPD (%, n) | 10% (19) | 13.8% (27) | 0.252 |
| Smoking (%, n) | 16.4% (31) | 10.7% (21) | 0.107 |
| Hypertension (%, n) | 12.6% (24) | 12.3% (24) | 0.908 |
| Dyslipidemia (%, n) | 12.6% (24) | 16.9% (33) | 0.244 |
| Creatinine (mg/dL) (mean |
1.03 |
1.02 |
0.725 |
| DM, Diabetes Mellitus; PRP, Platelet Rich Plasma; BMI, Body mass index; COPD, Chronic Obstructive Pulmonary Disease; IQR, interquartile range; SD, standard deviation. | |||
| PRP | No-PRP | |||
| Single pathogen infection | 8 | 14 | ||
| Staphylococcus aureus | 5 | 7 | ||
| MSSA | 1 | 2 | ||
| MRSA | 4 | 5 | ||
| Pseudomonas aeruginosa | 2 | 3 | ||
| Serratia marcescens | 1 | 3 | ||
| Acinetobacter baumanii | 0 | 1 | ||
| Polymicrobial infection | 7 | 10 | ||
| 2 pathogens | 5 | 4 | ||
| 3 pathogens | 2 | 5 | ||
| 4 pathogens | 0 | 1 | ||
| MSSA, Methicillin-sensitive Staphylococcus aureus; MRSA, Methicillin-resistant Staphylococcus aureus. | ||||
| Complications | PRP (n = 452) | No PRP (n = 501) | p value |
| Pain | 100 (22.1%) | 122 (24.3%) | 0.417 |
| Serous Exudate | 67 (14.8%) | 98 (19.5%) | 0.054 |
| Erythema | 66 (14.6%) | 71 (14.1%) | 0.850 |
| Purulent Exudate | 11 (2.4%) | 23 (4.5%) | 0.073 |
| Dehiscence | 13 (2.8%) | 24 (4.7%) | 0.127 |
| Infection | 16 (3.5%) | 26 (5.2%) | 0.215 |
| PRP, Platelet Rich Plasma. | |||
A logistic regression revealed a positive association between infection and COPD
(p = 0.038), smoking habit (p = 0.002), and diabetes mellitus (p = 0.002) (Table 4). We observed that comparing the diabetic patients in PRP group (PRP-DM) with
the diabetic patients in the control group (No PRP-DM) the rate of the SVHI was
lower in the treated one with statistically significance (PRP-DM: 2.6% vs
No-PRP-DM: 7.7%, p = 0.026). Likewise, in the diabetic patients, fewer major
complication of the wound healing process occurred in the treated group
(Table 5). Furthermore, the PRP-DM group showed a lower ASEPSIS score than the
control group (PRP-DM: 2.7
| Model | Deviance | AIC | BIC | df | Χ² | p | McFadden | Nagelkerke | Tjur | Cox & Snell | ||||||
| R² | R² | R² | R² | |||||||||||||
| H₀ | 344.365 | 346.365 | 351.224 | 952 | ||||||||||||
| H₁ | 303.007 | 321.007 | 364.743 | 944 | 41.358 | 0.120 | 0.140 | 0.066 | 0.042 | |||||||
| Coefficients | Wald test | |||||||||||||||
| Estimate | Standard Error | Odds Ratio | z | Wald Statistic | df | p | ||||||||||
| (Intercept) | –3.169 | 1.699 | 0.042 | –1.866 | 3.480 | 1 | 0.062 | |||||||||
| Age | –0.002 | 0.019 | 0.998 | –0.114 | 0.013 | 1 | 0.909 | |||||||||
| Sex | 0.074 | 0.385 | 1.077 | 0.192 | 0.037 | 1 | 0.848 | |||||||||
| BMI | –0.059 | 0.040 | 0.943 | –1.484 | 2.202 | 1 | 0.138 | |||||||||
| COPD | 0.769 | 0.370 | 2.157 | 2.080 | 4.325 | 1 | 0.038 | |||||||||
| Smoke | 1.545 | 0.487 | 4.689 | 3.173 | 10.069 | 1 | 0.002 | |||||||||
| Hypertension | 0.160 | 0.432 | 1.174 | 0.371 | 0.138 | 1 | 0.711 | |||||||||
| Dyslipidemia | 0.463 | 0.388 | 1.589 | 1.194 | 1.426 | 1 | 0.232 | |||||||||
| Diabetes | 1.159 | 0.381 | 3.188 | 3.040 | 9.239 | 1 | 0.002 | |||||||||
| BMI, Body mass index; COPD, Chronic Obstructive Pulmonary Disease; DM, diabetes
mellitus. Note: INFECTION level ‘Si’ coded as class 1. | ||||||||||||||||
| Complications | PRP-DM (n = 189) | No PRP-DM (n = 195) | p value | PRP-No-DM (n = 263) | No-PRP- No-DM (n = 308) | p value |
| Pain | 39 (20.6%) | 57 (29.2%) | 0.052 | 61 (23.1%) | 65 (21.1%) | 0.576 |
| Serous exudate | 24 (12.6%) | 53 (27.1%) | 43 (16.3%) | 45 (14.6%) | 0.589 | |
| Erythema | 16 (8.4%) | 30 (15.3%) | 0.037 | 50 (19%) | 41 (13.3%) | 0.069 |
| Purulent Exudate | 3 (1.58%) | 13 (6.6%) | 0.013 | 8 (3%) | 10 (3.2%) | 0.878 |
| Dehiscence | 5 (2.6%) | 15 (7.7%) | 0.026 | 8 (3%) | 9 (2.9%) | 0.944 |
| Infection | 5 (2.6%) | 15 (7.7%) | 0.026 | 11 (4.2%) | 11 (3.5%) | 0.717 |
| PRP, Platelet Rich Plasma; DM, Diabetes Mellitus. | ||||||
Leg wound healing after SV harvesting is an underestimated source of patient morbidity after cardiac surgery. The incidence of complications depends on the definition of wound infection and the follow-up frequency. The traditional open technique of SV harvesting provides an established and rapid way, with an optimal visualization of the vein. Unfortunately, this approach is associated with a large scar formation, unsatisfactory cosmetic result, and a considerable risk of wound infection. In 2005 Fowler reported a major infection of the saphenous harvest site that occurred in 32.6% of a population of 11,636 patients, after CABG [14]. Our total incidence of wound infection is 4.4%. Besides their role in hemostasis, platelets have been shown to be crucial in wound healing and immunomodulation. It is thus thought that autologous platelets at the site of tissue damage might enhance the healing process and thereby protect against infection [15]. Moreover, during the inflammatory phase of tissue healing, activated platelet releases specific growth factors which regulate the early migration of cells to the injury site, cell mitosis, angiogenesis, granulation tissue formation [16]. Because of these features, PRP, or platelet concentrate, has emerged as a possible adjuvant therapy to aid in the healing of surgical wounds and injuries. The benefit of autologous PRP application has been already appreciated to prevent the sternal wounds infections and for the treatment of left ventricular assist device driveline infections [10, 11, 12].
Englert and colleagues in their study included 30 patients and found a decreased wound bruising in PRP group [17]. Vang performed a study with 36 patients observing that PRP-treated patients experienced less postoperative pain, reduced blood loss, and reduced symptoms [18]. In 2008, a retrospective analysis of CABG patients having endoscopic vein harvesting [19] concluded that PRP significantly reduced occurrences of chest wound infection, chest and leg wound drainage, concluding that the PRP therapy merits further investigation. Recently, Almdhal and co-workers prospectively enrolled 140 patients concluding that the topical application of autologous platelet-rich plasma on vein harvest wounds did not reduce the rate of surgical site infection [20]. This randomized controlled trial enrolled a small number of patients, with low incidence of complications and so underpowered. Our findings suggest that the topical use of autologous PRP reduced the incidence of vein harvest wound infection following CABG. Moreover, the No-PRP group had a higher rate of microbiological isolation compared to the PRP group (No-PRP: 24 vs. PRP: 15, Table 2). As already mentioned by other studies, PRP could reduce the development of infections through the antimicrobial effect of white blood cells and platelets [12]. In vitro analyses showed different degrees of a potential antimicrobical action of PRP against several germs common in wounds such as Stapilococcus aureus (MRSA, MSSA), Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli, Enterococcus faecalis, Enterobacter cloacae, Proteus mirabilis, Acinetobacter baumanii, and Staphilococcus epidermidis. According to Farghali and colleagues, PRP has a bacteriostatic and bactericidal effect [21]. As like as antibiotics, PRP could counteract invading pathogens with a different spectrum of action. A minimum inhibitory concentration (MIC) should be reached also for PRP in order to overcome and stop the bacterial growth [22]. In the present paper, PRP could have both promoted the healing process and counteracted the potential strains of bacteria in the treated group, resulting in low wound infections and healing complications (Tables 2 and 3).
Moreover, our results suggest that diabetic patients might benefit from the topical application of PRP on the wound site, preventing the incidence of complications. Diabetes Mellitus is one of the major risk factors for poor wound healing. The low capillary oxygen delivery leads to wound infections at the harvest site. Numerous in vitro and in vivo studies demonstrate the beneficial effects of PRP in diabetic ulcers [23, 24]. Guo and colleagues observed the cutaneous healing process in chronic wounds treated with PRP in a diabetic rat model, providing evidence that platelet growth factor can effectively induce proliferation and migration of endothelial cells and fibroblasts to improve angiogenesis and re-epithelialization in chronic wounds [25]. Moreover, Chen and colleagues proved the antibacterial effect of autologous platelet-rich gel derived from subjects with diabetic dermal ulcers in vitro [26]. The application of platelet-rich gel was also found effective in enhancing healing of transmetatarsal amputations in diabetic dysvascular patients [27]. Massara and associates showed that the application of autologous platelet-rich plasma enhanced wound healing after lower limb revascularization [28]. Moreover, Ahmed and colleagues showed an increase in healing rate in PRP group vs. non-PRP group for the treatment of diabetic foot ulcers [29]. Our findings suggest that Staphylococcus aureus was the most common causal bacteria found in infected wounds, with a lower incidence in PRP group.
Despite close adherence to routine process of care measures, SVHI are an important source of morbidity in CABG patients. Although several risk factors (Diabetes Mellitus, smoking habit, obesity, etc.) can be identified, their impact on the rate of infection is not completely understood. A better understanding of patient risk factors, as well as the application of prevention strategies as like as PRP use, could guide future protocols of care.
Furthermore, the cost of the prophylactic application with PRP is certainly lower than that required for prolonged treatment of a SVHI (antibiotic administration, advanced wound dressing, vacuum-assisted closure therapy, in-hospital stay).
This study represents the largest retrospective comparison between PRP treated patients and control group for leg wound infections after cardiac surgery. The enrolled patients represent a standard CABG population. All baseline characteristics were well matched between the two groups. We exclude all the major confounding factors from our analysis as peripheral vascular disease, preoperative anemia, chronic renal failure. The overall care for patients was the same despite the PRP administration or the risk factors (including DM). Minor limitation is represented by the fact that all data were collected from one institution. Moreover, the diabetic population was not further studied considering the severity of the DM itself and the antidiabetic treatment.
Although adherence to basic surgical principles and proper vein harvest site selection still remain the essential factors in preventing leg wound complications, Platelet-Rich Plasma treatment seems to reduce the surgical site infection in diabetic patients. To better investigate its role, larger prospectively RCTs are required. Moreover, PRP impact of the new less invasive techniques and the incidence of harvest site infection in high-risk patients should be investigated.
CABG, Coronary artery bypass grafting; DM, Diabetes mellitus; GFs, growth factors; PRP, platelet-rich plasma; SV, saphenous vein; SVHI, saphenous vein harvest site infection.
FJ, RS, PM and GFS designed the research study. FJ, RS, AC, FV performed the research. MC and PM provided help and advice on methods. FJ, RS, AN analyzed the data. FJ, RS, AC, FV, PM, GFS wrote the manuscript. AN, FN, CC, MC revised the manuscript for significant intellectual content. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
IRB approval: ER.FE.2018.15.A — 11th September 2018, University Magna Graecia of Catanzaro.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
