Cucumeropsis mannii (CM) belongs to the melon family and is native to West Africa. There is a paucity of information on its medicinal or nutraceutical potential. Here, we examined the impact of CM in mice that were treated with a normal or a high fat diet (HFD). The CM extracts had a high levels of phenols, flavonoids, ascorbic acid and significant antioxidant activity. Treatment of mice with a HFD diet, led to the memory impairment. However, mice on HFD and received CM, despite increased food intake, showed a decrease in the body weight, locomotion, rearing, grooming, acetylcholinesterase activity and γ-amino butyric acid levels and anxiolysis. Also CM induced a reversal of HFD–induced changes in glucose levels, lipid peroxidation and super-oxide dismutase activity. These data show that CM leads to variable behavioural, biochemical and metabolic effects depending on the diet of animals.
Cucumeropsis mannii Naudin (C. mannii) is a plant belonging to the melon family which is native to tropical West, Central and East Africa. It is cultivated widely in a number of countries in West and central Africa, including Nigeria, Benin, and Cameroon (1-4). In these countries, it is generally cultivated for its seed (white melon or Mann’s cucumeropsis) which is used in making soups, or as a source of edible oil (4, 5). Cucumeropsis mannii seed is a rich source of minerals (including sodium, phosphorus, potassium, magnesium, calcium, iron, copper, manganese, selenium and zinc), carbohydrates and amino acids {histidine, isoleucine, leucine, lysine, methionine, cysteine, phenylalanine, tyrosine, threonine, tryptophan and Valine} (1, 4). Its protein content has been reported to be as high as 31-36% (4, 6), and C. mannii is also a rich source of fats, with reports showing a fat content as high as 52% and which its fatty acid component consists predominantly of linoleic acid, oleic acid, stearic acid and palmitic acid (4, 7). There have been suggestions that the high protein and nutrient composition of C. mannii make it of immense value in a number of the countries in sub-Saharan Africa where protein energy malnutrition amongst children and adults is fast becoming an epidemic due to poverty, drought and conflicts (8-11). Also, the high cost of animal sources of protein in this region has led to advocacy for the incorporation of plant-based protein and a relatively cheap source of amino acids like C. mannii into diet (4, 12). While there are potential benefits to be derived from the high protein content of C. mannii, the possible effects of its high fat/fatty acid composition is yet to be extensively evaluated. In a number of the countries where cucumeropsis mannii is consumed as a soup, fats like palm oil is added to increase its palatability; also when it is consumed as a cake, it is first defatted and then fried either in its oil or in oil from another vegetable source. However, both methods of processing for consumption have the potential to alter lipid profile, leading to either potentially beneficial or deleterious effects if consumed in large quantities.
The health benefits and medicinal value of a number of the closely-related members of the Cucurbitaceae family including Citrullus colocynthis (13, 14), Citrullus lanatus (15-17), Lagenaria siceraria (18, 19), Momordica charantia (20, 21) have been reported. However, beyond the direct value of Cucumeropsis mannii as a rich source of nutrients, research is yet to examine its atherogenicity or its medicinal value, especially its possible role in the prevention or management of dyslipidaemia. Therefore, this study examined the effects of C. mannii (incorporated into either standard diet or high-fat diet) on changes in body weight, neurobehaviour, blood glucose levels, lipid chemistry, antioxidant status and biochemical parameters of liver and kidney function in mice. We tested the hypothesis that diet-incorporated C. mannii could significantly alter metabolic profile, antioxidant status, and biochemical measures of liver/kidney function in mice.
All chemicals used were analytical standards (purity 98%), and purchased from Sigma–Aldrich, United Kingdom.
Dried cucumeropsis mannii seeds were sourced (from Osogbo, Osun State, Nigeria), shelled, screened and ground (Figure 1) into powder with an electric blender. Petroleum ether and ethanol extracts of powdered cucumeropsis mannii was used for qualitative phytochemical analysis as described by Sani (22), while for the in vivo tests, powdered C. mannii was incorporated into rodent chow (Standard diet or high-fat diet). Acute oral toxicity tests were carried out according to the Organization for Economic Co-operation and Development (OECD) guidelines (23).
 Figure 1
Figure 1Showing Cucumeropsis mannii seed and powdered Cucumeropsis mannii
Phytochemical screening and quantitative analysis of phytochemical constituents was carried out on three extracts (aqueous, petroleum-ether and methanol) of cucumeropsis mannii using standard procedures and tests (22, 24-26).
The total phenolic content of C. mannii was determined by mixing aliquots (1.0 ml) of each extract with equal volume of water and 2.5 ml Folin-Ciocalteu’s reagent. The mixture was neutralized with 2 ml of 7.5% sodium carbonate. Following which the mixture was incubated at 45 °C for 40 min, and the absorbance measured at 765 nm in the spectrophotometer. The total phenolic content was subsequently calculated as gallic acid equivalent (26).
The flavonoids content of C. mannii was determined using quercetin as standard. Briefly, O.5 ml of stock solution of extract was mixed with 0.5 ml methanol, 50 μL of 1 M potassium acetate, 50 μL of 10% Aluminium chloride and 1.4 ml water. The mixture was incubated at room temperature for 30 min, following which the absorbance was measured at 415 nm. The flavonoid content of C. mannii was expressed as quercetin equivalent (QE).
The ascorbic content of the samples was determined using ascorbic acid as a standard. This standard was prepared by dissolving 2mg/ml of ascorbic acid in water. 300μl of the sample preparation was mixed with 100μl of 13% TCA and 75μl of DNPH (dinitrophenylhydrazine), the resultant solution were incubated in a water bath for 3 hours at 37 oC, following 500μl of 65% H2SO4 (25)
The antioxidant capacity of C. mannii extracts was determined using the 1, 1-diphenyl–2 picrylhydrazyl (DPPH) scavenging assay (26). The DPPH assay is used to evaluate the ability of compounds to scavenge free radicals which is a major factor in biologic injury as a result of oxidative stress. Diluted extracts (1 ml) was mixed with 3 ml of 0.4 mM DPPH methanolic solution. The resulting mixture was incubated in the dark for 30 min and the absorbance measured at 516 nm. The DPPH free radical scavenging ability was then calculated.
All procedures performed on the animals were in accordance with approved protocols of the Ladoke Akintola University of Technology and within the provisions for animal care and use as prescribed by the scientific procedures on living animals, European Council Directive (EU2010/63).
Healthy male Swiss mice obtained from Empire Breeders, Osogbo, Osun State, South-West Nigeria were used for the in-vivo tests. Mice were housed in plastic cages measuring 12 x 9 x 6 inches. General housing was in a temperature-controlled (22.5°C ±2.5°C) quarters with 12 hours of light. Mice had free access to food and water. At the beginning of the experiments, mice were housed singly to allow the estimation of food and water intake. All procedures were conducted in accordance with the approved institutional protocols and within the provisions for animal care and use prescribed in the scientific procedures on living animals, European Council Directive (EU2010/63).
All animals were fed rodent chow from weaning. At the beginning of the experimental period, animals were either fed standard chow (29% protein, 11% fat, 58% carbohydrate) or high-fat diet (18% protein, 42% fat, 36% carbohydrate) which was compounded from palm olein and vegetable shortening (hydrogenated), as previously reported (27). Powdered C. mannii was incorporated into standard or high-fat diet at 5 (50 g/Kg of feed), 10 (100 g/Kg of feed) and 20 (200 g/Kg of feed) percent., based on the results of acute toxicity test and a previous study by Mbuli-Lingundi et al. (28) that reported that consumption of 100 g of dehulled seeds sufficiently covered the daily requirement of essential fatty acids, and essential amino acids. Diet was administered ad libitum for a period of eight weeks.
Eighty male, young adult mice weighing between 20-25 g each were randomly-assigned into eight groups of ten (n=10) animals each. Mice were grouped as follows (Table 1), control (standard diet), high-fat control (High-fat diet), three groups of C. mannii incorporated into standard diet and three groups of C. mannii incorporated into high fat diet at 50 (5 %), 100 (10 %) and 200 (20 %) g/Kg of food. Body weight and food intake was measured using a weighing balance (Mettler Toledo Type BD6000, Greifensee, Switzerland). Animals were fed standard diet (SD), high fat diet (HFD) or cucumeropsis mannii incorporated diet daily for a period of eight weeks. Behavioural tests (in the Open field, Y-maze, radial-arm maze and elevated plus maze) were conducted on day 56 (because treatment for 8 weeks in our laboratory has been associated with the development of metabolic derangement) (27). Twenty-four hours after the last behavioural test (day 57), blood was taken from the tail vein after an overnight fast for estimation of glucose levels by the glucose oxidase method (29, 30). Mice in all groups were then euthanised by cervical dislocation. Blood taken via an intra-cardiac puncture was used for estimation of lipid profile, biochemical parameters of liver/renal integrity, and lipid peroxidation levels. The liver, kidney and brain were dissected, weighed and homogenised for assessment of superoxide dismutase activity, while whole brain homogenate was also used to assay for acetylcholinesterase activity and GABA levels.
| Groups | SD | HFD | Behavioural /Biochemical tests |
|---|---|---|---|
| Control | Yes | No | Yes |
| CM 5 | Yes | No | Yes |
| CM 10 | Yes | No | Yes |
| CM 20 | Yes | No | Yes |
| HFD | No | Yes | Yes |
| HFD/ CM 5 | No | Yes | Yes |
| HFD/ CM 10 | No | Yes | Yes |
| HFD/ CM 20 | No | Yes | Yes |
Mice were tested in the Y-maze (5 min), open-field (10 min) and radial-arm maze (5 min). Animals were placed individually in the arena and their behaviours recorded, following which they were put back in their home cages. The mazes were cleaned with 70 % ethanol and wiped dry after testing each animal. Two independent observers with no knowledge of the treatment administered scored the behaviours.
In the open-field, horizontal locomotion, rearing and grooming behaviours were assessed and scored as previously described (31, 32). Open-field dimension was 36 x 36 x 26 cm and its floor was divided into 16 equal squares.
The Y- and the radial arm- maze were used to assess and score spatial working-memory as previously described (33, 34). Each mouse was placed in one of the three arms of the Y-maze or an 8-arm radial arm maze apparatus (with each arm measuring 33 cm in length) and allowed to explore all arms.
The EPM is validated for the assessment of anxiety-related behaviours in mice. It has four arms, two open arms measuring 25 x 5 x 0 cm lying across from each other and perpendicular to two closed arms measuring 25 x 5 x 16 cm with a centre platform. Each mouse was allowed to explore the arms for 5 minutes while time spent in the open and closed arms were scored as previously described (35).
Blood was collected from each mouse on the 57th day via intra-cardiac puncture after an overnight fast. Samples were collected into universal bottles, allowed to clot, centrifuged at 3,500 rpm for 10 minutes using a g0eneral centrifuge (Uniscope SM112, Surgifriend Medicals, England) to allow separation as previously described (36, 37). The serum was assayed either immediately or stored at -20 oC.
Within 24 hours of the completion of the behavioural tests, animals were sacrificed by cervical dislocation. Liver, kidney and brains were dissected, blotted dry and weighed. A 10 % homogenate of each of the organs was prepared with ice-cold phosphate buffered saline, using a Teflon-glass homogeniser. The homogenate was thereafter centrifuged at 5,000 rpm (4 °C) for 15 min, and used for biochemical analysis.
Alanine transaminase (ALT), aspartate transaminase (AST) were assayed using the colorimetric method by measuring concentration of oxaloacetate hydrazone and the pyruvate hydrazone formed with 2,4-dinitrophenyl-hydrazine, respectively; colour change was measured at 546 nm (30, 38, 39).
Total cholesterol, triglycerides, HDL-C and LDL-C in serum were also analysed using commercially available kits following the instructions of the manufacturer.
Superoxide dismutase activity was assayed from tissue homogenates as previously described (40-42). Superoxide dismutase assay is based on the principle of the enzyme’s ability to inhibit phenazine methosulphate-mediated reduction of the nitro blue tetrazolium dye. The colour change was measured at an absorbance of 560 nm over 5 minutes.
The level of malondialdehyde (MDA) in samples was used to assess the degree of lipid peroxidation as previously described (43, 44). Reactive substances of the thiobarbituric acid react with free MDA in tissue to produce a coloured complex (TBAR-MDA adducts) which is measured at an absorbance of 532 nm. The MDA concentration is expressed as nmol/g of tissue.
Acetylcholinesterase activity of the brain homogenate was measured using acetylcholinesterase colorimetric assay kit, according to the instructions of the manufacturer. Colour change was measured at an absorbance of 410 nm, and results were expressed as units per microgram of total protein (U/mg).
Gamma-amino-butyric acid levels of brain homogenate were assayed using commercially-available assay kit following the instructions of the manufacturer. Colour change was measured at an absorbance of 450 nm. The assay kit had a detection range of between 6 and 400 pg/ml and a sensitivity of < 1.2 pg/m
Data was analysed using Chris Rorden’s ezANOVA for windows. Hypothesis was tested using analysis of variance (ANOVA). We tested the hypothesis that Cucumeropsis mannii supplemented diet can significantly alter body weight, food intake, neurobehaviour, blood glucose, antioxidant status, lipid profile, lipid peroxidation status, and biochemical parameters of hepatic function in mice fed normal or high fat diet. One factor ANOVA was used to test effects of diet on these parameters. Tukey (HSD) test was used for post-hoc analysis. Results were expressed as mean ± S.E.M, and p< 0.05 considered significant.
Table 2 shows the results of phytochemical analysis of the aqueous, petroleum ether and methanol extracts of powdered cucumeropsis mannii seeds. All extracts tested positive for sterols, flavonoids, phenols, alkaloids and tannins, while only the methanolic extract was positive for reducing sugars. Table 3 shows the result of total phenolic flavonoid content and DPPH scavenging ability of the C. mannii extracts. The highest phenolic and flavonoid content were observed with the methanolic extract, while the petroleum ether extract had the lowest phenolic and flavonoid content and antioxidant activity. DPPH inhibitory activity was highest with the aqueous extract and lowest with the petroleum ether extract.
| Compounds | Aqueous Extract | Methanol extract | Petroleum ether extract |
|---|---|---|---|
| Anthraquinones | - | - | - |
| Sterols | + | + | + |
| Flavonoids | + | + | + |
| Phenols | + | + | + |
| Alkaloids | + | + | + |
| Anthocyanins | + | - | - |
| Phytate | + | + | + |
| Oxalate | + | + | + |
| Tannins | + | + | + |
| Saponins | + | - | - |
| Reducing sugars | + | + | - |
| Samples | Total phenolic content (mg GAE/g) | Total flavonoid content (mg QE/g) | DPPH (IC50μg/ml) | Ascorbic acid (mg/g) |
|---|---|---|---|---|
| Aqueous | 0.05±0.02 | 0.05±0.01 | 78.3 ± 1.10 | 101±0.02 |
| Petroleum ether | 0.03±0.01 | 0.03±0.01 | 82.33±1.20 | 0.50±0.02 |
| Methanol | 0.10±0.02 | 0.12±0.01 | 72.3± 1.16 | 0.56±0.02 |
Figure 2 shows the effect of cucumeropsis mannii (CM) on body weight (upper panel) and food intake (lower panel). There was a significant (F (7, 72) = 42.5, p < 0.001) increase in body weight in the groups fed CM at 5, 10 and 20 %, HFD, and HFD/CM at 5 and 10 % compared to control. Compared to HFD, there was a significant decrease in body weight with HFD/CM at 10 and 20 %. Intra-group comparisons revealed a significant decrease in body weight with HFD/CM at 20 % compared to respective group of mice fed CM.
 Figure 2
Figure 2Effect of Cucumeropsis mannii on change in body weight (upper panel) and food intake (lower panel). Each bar represents Mean ± S.E.M, *p < 0.05 vs. control, #p < 0.05 vs. HFD, &p < 0.05 CM alone CM /HFD. Number of mice per treatment group =10. HFD: High fat diet, CM: Cucumeropsis mannii
Food intake decreased significantly (F (7, 72) = 11.2, p < 0.04) with HFD/CM at 5% and increased significantly with HFD/CM at 10 and 20 % compared to SD control. Compared to HFD, food intake increased significantly with HFD/CM at 10 and 20%. Intra-group comparisons revealed a significant decrease in food intake with HFD/CM at 5% and an increase at 10 and 20 % compared to respective group of mice fed SD/CM.
Figure 3 shows the effect of CM on locomotor activity (upper panel), rearing (lower panel). There was a significant ( (F (7, 72) = 7.50, p < 0.010) decrease in locomotor activity in the groups fed CM alone at 5, 10 and 20 %, HFD alone and HFD/CM at 5, 10 and 20 % compared to control (standard diet). Compared to HFD, there was a significant increase in locomotor activity with HFD/CM at 10 and 20%. Intra-group comparisons revealed a significant decrease in locomotor activity with HFD/CM at 5 and 20 %, compared to respective groups of mice fed CM alone.
 Figure 3
Figure 3Effect of Cucumeropsis mannii on locomotor (upper panel) and rearing (lower panel) activity. Each bar represents Mean ± S.E.M, *p < 0.05 vs. control, #p < 0.05 vs. HFD, &p < 0.05 CM alone CM /HFD. Number of mice per treatment group =10. HFD: High fat diet, CM: Cucumeropsis mannii
Rearing decreased significantly (F (7, 72) = 25.6, p < 0.001) with CM at 10 and 20 %, HFD, and HFD/CM at 5, 10 and 20 % compared to standard diet control. Compared to HFD, there was an increase in rearing with HFD/CM at 5, 10 and 20 %. Intra-group comparisons revealed a significant decrease in rearing with HFD/CM at 5% and a significant increase in rearing with HFD/CM at 10 and 20 % compared to respective groups of mice fed CM.
Figure 4 shows the effect of CM on self grooming. Self-grooming decreased significantly (F (7, 72) = 7.11, p < 0.016)) with CM at 10 and 20 %, HFD alone and HFD/CM at 5, 10 and 20 % compared to SD control. Compared to HFD, grooming decreased with HFD/CM at 10 and 20 %. Intra-group comparisons revealed a significant decrease in grooming with HFD/CM at 5 and 10 %, compared to respective groups of mice fed CM.
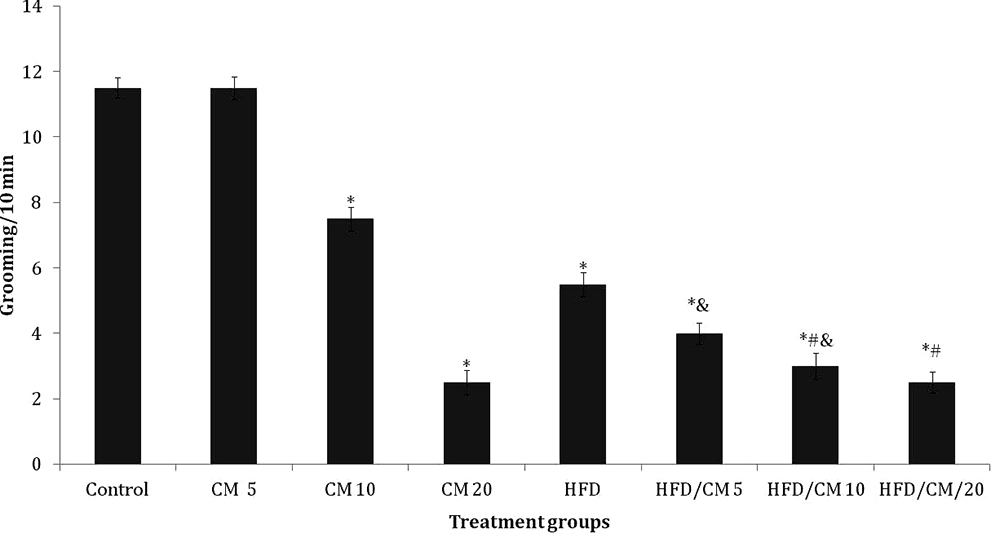 Figure 4
Figure 4Effect of Cucumeropsis mannii on self-grooming. Each bar represents Mean ± S.E.M, *p < 0.05 vs. control, #p < 0.05 vs. HFD, &p < 0.05 CM alone CM /HFD. Number of mice per treatment group =10. HFD: High fat diet, CM: Cucumeropsis mannii.
Figure 5 shows the effect of CM on Y-maze spatial working memory (upper panel), and radial-arm working memory (lower panel). There was a significant ( (F (7, 72) = 7.38, p < 0.011) decrease in Y-maze working memory in the groups fed CM at 20 %, HFD, and HFD/CM at 5, 10 and 20 % compared to SD control. Compared to HFD, there was a significant increase in working memory with HFD/CM at 5, 10 and 20%. Intra-group comparisons revealed no significant difference in working memory with HFD/CM compared to respective groups of mice fed CM.
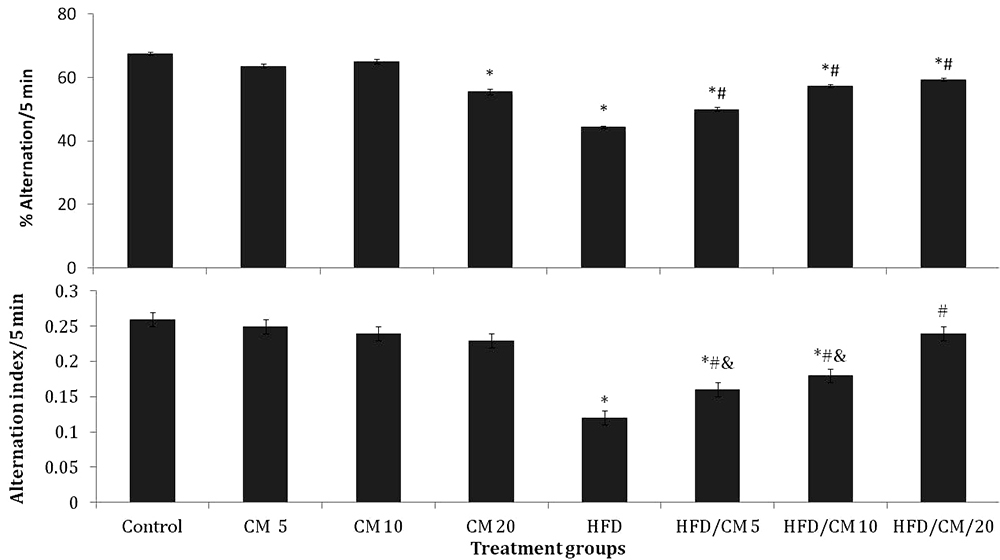 Figure 5
Figure 5Effect of Cucumeropsis mannii on Y-maze spatial working memory (upper panel) and radial-arm maze spatial working memory (lower panel). Each bar represents Mean ± S.E.M, *p < 0.05 vs. control, #p < 0.05 vs. HFD, &p < 0.05 CM alone CM /HFD. Number of mice per treatment group =10. HFD: High fat diet, CM: Cucumeropsis mannii.
Radial-arm spatial working memory decreased significantly (F (7, 72) = 6.98, p < 0.027) with HFD and HFD/CM at 5 and 10 % compared to SD control. Compared to HFD, radial-arm spatial working memory increased with HFD/CM at 20 %. Intra-group comparisons revealed a significant decrease in radial-arm working memory with HFD/CM at 5 and 10 % compared to respective groups of mice fed CM.
Figure 6 shows the effect of CM on time spent in the open arm (Upper panel) and closed arm (lower panel) of the elevated plus maze. Open arm time increased significantly (F (7, 70) = 17.32, p < 0.001)) with CM at 5, 10 and 20 % and HFD/CM at 5, 10 and 20 %; while it decreased with HFD compared to SD control. Compared to HFD, open arm time increased with HFD/CM at 5, 10 and 20 %. Intra-group comparisons revealed no significant difference in open arm time with HFD/CM compared to respective groups of mice fed CM.
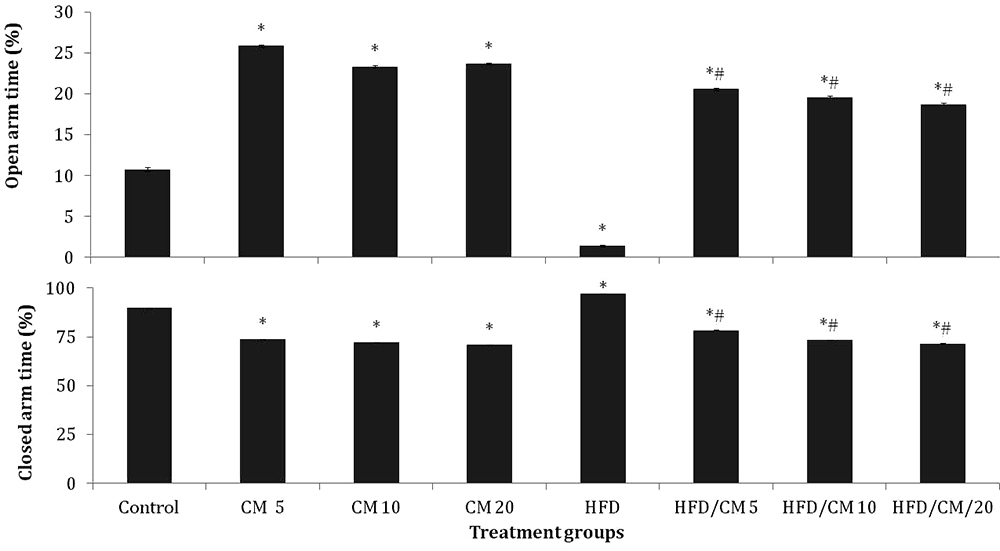 Figure 6
Figure 6Effect of Cucumeropsis mannii on time spent in the open arm (upper panel) and closed arm (lower panel) of the elevated plus maze. Each bar represents Mean ± S.E.M, *p < 0.05 vs. control, #p < 0.05 vs. HFD, &p < 0.05 CM alone CM /HFD. Number of mice per treatment group =10. HFD: High fat diet, CM: Cucumeropsis mannii.
Closed arm time decreased significantly (F (7, 72) = 8.02, p < 0.020)) with CM alone at 5, 10 and 20 %, and HFD/CM at 5, 10 and 20 %, while it increased significantly with HFD compared to control. Compared to HFD, time spent in the closed arm decreased significantly with HFD/CM at 5, 10 and 20 %. Intra-group comparisons revealed no significant difference in closed arm time with HFD/CM compared to respective groups of mice fed CM alone.
Table 4 shows the effect of CM on blood glucose levels, and biochemical parameters of liver and kidney function. Blood glucose levels increased significantly (F (7, 72) = 64.5, p < 0.001) with CM at 5, 10 and 20 %, HFD, and HFD/CM at 5, 10 and 20 % compared to SD. Compared to HFD, there was a significant decrease in blood glucose with HFD/CM at 5, 10 and 20 %. Intra-group comparisons revealed no significant difference in blood glucose levels with HFD/CM compared to respective groups of mice fed CM alone.
| Groups | Glucose (mg/dl) | AST (mmol/L) | ALT (mmol/L) | Urea (mmol/L) | Creatinine (mmol/L) |
|---|---|---|---|---|---|
| Control | 85.33 ±0.42 | 87.60±3.51 | 26.17±0.70 | 2.82±0.10 | 67.67±3.58 |
| CM 5 | 106.5±1.591 | 86.33±2.15 | 28.27±1.15 | 2.51±0.25 | 68.10±2.40 |
| CM 10 | 107.5±0.671 | 89.17±2.14 | 24.20±0.17 | 2.90±0.20 | 70.60±2.20 |
| CM 20 | 115.5±1.011 | 81.13±2.36 | 27.67±1.25 | 3.01±0.20 | 72.17±1.20 |
| HFD | 167.0±0.451 | 205.10±2.121 | 57.33±1.251 | 5.22±0.201 | 100.67±3.30* |
| HFD/CM 5 | 100.33±1.401,2 | 132.15±2.121,2,3 | 58.27±0.301,3 | 2.22±0.101,2 | 83.34±2.601,2,3 |
| HFD/CM 10 | 110.50±1.461,2 | 129.65±3.111,2,3 | 33.67±1.201,2 | 2.97±0.201,2 | 80.60±2.201,2,3 |
| HFD/CM 20 | 116.0±0.891,2 | 179.17±1.331,2,3 | 31.10±1.501,2 | 3.27±0.101,2 | 82.60±2.501,2,3 |
Aspartate transaminase (AST) levels increased significantly (F (7, 72) = 15.2, p < 0.001) with HFD, and HFD/CM at 5, 10 and 20 % compared to SD control. Compared to HFD, there was a significant decrease in AST levels with HFD/CM at 5, 10 and 20 %. Intra-group comparisons revealed a significant increase in AST levels with HFD/CM at 5, 10 and 20 % compared to respective groups of mice fed CM.
Alanine transaminase (ALT) levels increased significantly (F (7, 72) = 10.1, p < 0.001) with HFD, and HFD/CM at 5, 10 and 20 % compared to SD control. Compared to HFD, there was a significant decrease in ALT levels with HFD/CM at 10 and 20 %. Intra-group comparisons revealed a significant increase in ALT levels with HFD/CM at 5 % compared to respective groups of mice fed CM.
Urea levels increased significantly (F (7, 72) = 9.1, p < 0.002) with HFD, and HFD/CM at 5, 10 and 20 % compared to control. Compared to HFD, there was a significant decrease in urea levels with HFD/CM at 5, 10 and 20 %. Intra-group comparisons revealed no significant difference in urea levels with HFD/CM compared to respective groups of mice fed CM alone.
Creatinine levels increased significantly (F (7, 40) = 4.5, p < 0.041) with HFD, and HFD/CM at 5, 10 and 20 % compared to control. Compared to HFD, there was a significant decrease in creatinine levels with HFD/CM at 5, 10 and 20 %. Intra-group comparisons revealed a significant increase in creatinine levels with HFD/CM at 5, 10 and 20 % compared to respective groups of mice fed CM alone.
Table 5 shows the effect of CM on lipid profile and atherogenic index. Total cholesterol (TC) increased significantly (F (7, 72) = 62.7, p < 0.001) with CM alone at 5, 10 and 20 %, HFD, and HFD/CM at 5, 10 and 20 % compared to control. Compared to HFD, there was a significant increase in TC with HFD/CM at 5, 10 and 20 %. Intra-group comparisons revealed a significant decrease in TC levels with HFD/CM at 5, 10 and 20 % compared to respective groups of mice fed CM alone.
| Groups | TC (mmol/L) | TG (mmol/L) | LDL (mmol/L) | HDL (mmol/L) | LDL/HDL ratio | MDA μM |
|---|---|---|---|---|---|---|
| Control | 1.47±0.04 | 0.55±0.10 | 0.90±0.04 | 0.37±0.03 | 2.17±0.21 | 1.52±0.01 |
| CM 5 | 10.80±0.591 | 2.67±0.111 | 2.00±0.021 | 1.03±0.02* | 1.94±0.21 | 1.62±0.02 |
| CM 10 | 5.43±0.241 | 2.80±0.171 | 1.76±0.371 | 0.93±0.16* | 1.90±0.31 | 1.55±0.04 |
| CM 20 | 6.93±0.751 | 2.13±0.151 | 1.83±0.161 | 0.83±0.20* | 2.20±0.41 | 1.85±0.02 |
| HFD | 2.10±0.111 | 1.10±0.131 | 1.38±0.061 | 0.25±0.04* | 5.55± 0.211 | 4.78±0.131 |
| HFD/CM 5 | 4.37±0.041,2,3 | 1.40±0.101,2 | 1.50±0.181,2 | 2.00±0.13*# | 0.75±0.191,2,3 | 1.18±0.111,2,3 |
| HFD/CM 10 | 3.18±0.111,2,3 | 2.00±0.121,2 | 1.23±0.181,2 | 1.45±0.02*# | 0.86±0.111,2,3 | 1.37±0.031,2,3 |
| HFD/CM 20 | 3.30±0.051,2,3 | 2.55±0.111 | 1.72±0.121,2 | 1.45±0.20*# | 1.25±0.211,2,3 | 2.65±0.121,2 |
Triglyceride (TG) levels increased significantly (F (7, 72) = 98.4, p < 0.001) with CM at 5, 10 and 20 %, HFD and HFD/CM at 5, 10 and 20 %, compared to control. Compared to HFD, there was a significant increase in TG with HFD/CM at 10 and 20 %. Intra-group comparisons revealed no significant difference in TG with HFD/CM compared to respective groups of mice fed CM alone.
Low density lipoprotein (LDL) levels increased significantly (F (7, 72) = 63.8, p < 0.001) with CM at 5, 10 and 20 %, HFD and HFD/CM at 5, 10 and 20 %, compared to control. Compared to HFD, there was a significant increase in LDL with HFD/CM at 5, 10 and 20 %. Intra-group comparisons revealed no significant difference in LDL with HFD/CM compared to respective groups of mice fed CM alone.
High density lipoprotein (HDL) levels increased significantly (F (7, 72) = 30.6, p < 0.001) with CM at 5, 10 and 20 %, and HFD/CM at 5, 10 and 20 %; and decreased significantly with HFD compared to control. Compared to HFD, there was a significant increase in HDL with HFD/CM at 5, 10 and 20 %. Intra-group comparisons revealed no significant difference in HDL levels with HFD/CM compared to respective groups of mice fed CM alone.
LDL/HDL ratio increased significantly (F (7, 72) = 4.25, p < 0.001) with CM at 5, 10 and 20 % and HFD and decreased with HFD/CM at 5, 10 and 20 % compared to control. Compared to HFD, there was a significant decrease in LDL/HDL ratio with HFD/CM at 5, 10 and 20 %. Intra-group comparisons revealed a significant decrease in LDL/HDL ratio with HFD/CM at 5, 10 and 20 % compared to respective groups of mice fed CM alone.
Malondialdehyde (MDA) levels increased significantly (F (7, 72) = 32.1, p < 0.001) with HFD and HFD/CM at 20 %, while MDA levels decreased with HFD/CM at 5 and 10% compared to control. Compared to HFD, there was a significant decrease in MDA levels with HFD/CM at 5, 10 and 20 %. Intra-group comparisons revealed a significant decrease in MDA levels with HFD/CM at 5 and 10 % compared to respective groups of mice fed CM alone.
Table 6 shows the effect of CM on superoxide dismutase (SOD) activity in homogenates of the liver, kidney and brain. SOD activity increased significantly in the liver (F (7, 72) = 12.1, p < 0.001), Kidney (F (7, 72) = 8.1, p < 0.011) and whole brain (F (7, 72) = 4.1, p < 0.011) with CM at 5, 10 and 20 %, HFD and HFD/CM at 5, 10 and 20 %, compared to control. Compared to HFD, there was a significant decrease in SOD activity with HFD/CM at 5, 10 and 20 %. Intra-group comparisons revealed no significant difference in SOD activity with HFD/CM compared to respective groups of mice fed CM alone.
| Groups | Liver SOD U/mg/protein | Kidney SOD U/mg/protein | Brain SOD U/mg/protein | Brain ACHe nmol/mg | Brain GABA (μmol/g) |
|---|---|---|---|---|---|
| Control | 1.59±0.04 | 1.41±0.01 | 1.30±0.11 | 22.10±1.10 | 9.0±1.08 |
| CM 5 | 2.12±0.021 | 1.94±0.021 | 1.70±0.101 | 23.20±1.10 | 12.10±1.191 |
| CM 10 | 2.15±0.011 | 1.99±0.011 | 1.95±0.111 | 22.10±1.40 | 14.43±1.071 |
| CM 20 | 2.35±0.021 | 2.10±0.011 | 2.12±0.101 | 21.20±1.20 | 14.65±1.101 |
| HFD | 7.70±0.231 | 5.21±0.141 | 4.10±0.201 | 35.20±1.421 | 6.21±1.13 |
| HFD/CM 5 | 3.14±0.101,2 | 2.11±0.101,2 | 2.24±0.311,2 | 30.22±2.101 | 12.61±1.351,2 |
| HFD/CM 10 | 3.22±0.111,2 | 2.15±0.031,2 | 2.33±0.431,2 | 33.10±1.351 | 12.8±1.151,2 |
| HFD/CM 20 | 4.24±0.161,2 | 2.43±0.111,2 | 2.55±0.501,2 | 28.20±1.641 | 13.42±1.151,2 |
Brain acetylcholinesterase (ACHe) activity increased significantly (F (7, 72) = 8.18, p < 0.031) with HFD and HFD/CM at 5, 10 and 20 % compared to standard diet control. Compared to HFD, ACHe activity did not differ significantly in any of the groups fed HFD/CM. Intra-group comparisons revealed no significant difference in ACHe activity with HFD/CM compared to respective groups of mice fed CM alone.
Brain GABA levels decreased significantly (F (7, 72) = 21.24, p < 0.001) with HFD and increased with CM at 5, 10 and 20 % and HFD/CM at 5, 10 and 20 % compared to standard diet control. Compared to HFD, GABA levels increased with HFD/CM at 5, 10 and 20 %. Intra-group comparisons revealed no significant difference in GABA levels with HFD/CM compared to respective groups of mice fed CM alone.
This present study (Figure 7) examined the effects of Cucumeropsis mannii incorporated into normal or high-fat diet on changes in body weight, food intake, neurobehaviour, lipid profile, biochemical parameters of liver and kidney integrity, oxidative stress parameter, brain acetylcholinesterase activity and brain GABA levels. The results showed that compared to control diet fed mice, C. mannii was associated with an increase in body weight, increase in food intake in mice fed HFD/CM; and a reduction in open field locomotion/rearing/grooming behaviours, anxiolysis and cognitive impairment in mice fed HFD/CM. Cucumeropsis mannii was also associated with an euglycaemic effect on blood glucose levels, alteration in lipid parameters and LDL/HDL ratio, as well as a decrease in lipid peroxidation and increase antioxidant activity. Brain acetylcholinesterase levels increased in groups fed HFD/CM while GABA levels increased with CM irrespective of dietary composition.
 Figure 7
Figure 7Graphical abstract showing the different methods employed in this study to evaluate the medicinal value of Cucumeropsis mannii.
Researchers have described the existence in Nigeria of at least six species of the melon plant including Cucumeropsis mannii, Citrullus lanatus, Cucurbita maxima, Citrullus vulgari, Cucumis melo L. and Cucumis melo var agrestis (45-47). A number of these species including Citrulus lanatus, Citrulus vulgaris, and cucumis melo var agrestis have been examined (48-53), with suggestions that the consumption of food derived from these plants have the ability to alter body weight, with possible therapeutic benefits in obesity (48, 52, 53) There is however a dearth of scientific information on the effects of C. mannii on the brain.
In this study, feeding mice with CM was associated with a concentration dependent decrease in weight gain. The highest level of weight gain observed in mice fed CM with standard diet and the lowest weight gain observed in group of mice fed HFD/CM. However, in contrast, a concentration dependent increase in food intake was observed in mice fed HFD/CM. Ajayi and Salami (54) examining the effect of C. mannii on body weight reported no change in body weight or food intake in rats fed defatted C. mannii (compared to control); and while Achu et al (55) reported that in rats fed C. mannii oil incorporated into diet, a 132% increase in body weight was observed, this was not compared against standard diet control. However, the results of these two studies (54, 55) suggest that the presence of fats in C. mannii also impacts weight gain. Overall, the weight reduction observed with C. mannii, especially in the groups fed HFD/CM could be attributed to either an increase in energy expenditure, reduced feed conversion, (especially when an increase in food intake was observed in these same groups), and possibly the anti obesity effect of fatty acid components of C. mannii. Studies (56-59) have associated a diet rich in plant sterols and polyunsaturated fatty acid with a reduction in cholesterol levels (especially the low density cholesterol), and an increase in energy expenditure and lipogenesis (60, 61).
Apart from the effects on body weight and food intake, an increase in total cholesterol, triglycerides, LDL and HDL cholesterol was observed in all groups fed CM compared to control; although TC, LDL levels was significantly lower and HDL higher in the groups fed HFD/CM compared to CM alone or mice fed HFD. Cucumeropsis mannii has been reported to contain both saturated and unsaturated fatty acids, with its saturated fatty acid content reported to be higher than its unsaturated fatty acid content (55); although another study has reported a contrary opinion regarding their relative composition (4). Studies evaluating the fatty acid content of cucumeropsis mannii grown in Nigeria (where the C. mannii variety used in this study was grown) reported that the predominant fatty acid in the seed oil was linoleic and oleic acids (56, 57). While fatty acid composition was not assessed in this study, the results of qualitative phytochemical analysis of C. mannii used in this study revealed presence of sterols, which is consistent with studies that had reported that C. mannii has a high content of sterols including campesterol, β-sitosterol and stigmasterol (28, 55, 56). There have been reports that linoleic acid is the most abundant unsaturated fatty acid found in C. mannii (4, 55, 56). The lipid lowering effect (at least when incorporated into HFD) could possibly be attributable to the linoleic acid content of C. mannii. Linoleic acid is an important essential fatty acid which is crucial to proper growth and maintenance of body physiology. Linoleic acids have also been associated with anti-obesity effects through their ability to increase energy expenditure, inhibit adipogenesis and suppress lipogenesis (61).
Also, in this study administration of CM to control animals was associated with a slight increase in blood glucose levels compared to animals fed control diet, although the values observed were within normal range of blood glucose observed in our laboratory (78-120 mg/dl). In animals fed HFD alone, a derangement in blood glucose levels was observed which was reversed in groups fed HFD/CM. From this study Cucumeropsis mannii can be said to exert an euglycaemic effect, and this could be attributed to its phenolic and flavonoid content. Studies have reported the antihyperglycaemic potential of phenols and flavonoids in diet (36, 37, 62). In this study, quantitative phytochemical analysis revealed the highest phenolic and flavonoid content in the aqueous extract of C. mannii; also another study that examined the phenolic and flavonoid composition of aqueous extract of CM reported the presence of high quantities of quercetin and luteolin, and the presence of appreciable quantities of rutin and quercetin (26).
Overall, the metabolic effects on C. mannii when incorporated into standard control diet included concentration–dependent weight reduction, hypercholesterolaemia and normoglycaemia/euglycaemia; however, in the groups fed HFD/CM there was a reversal of the deleterious effects of HFD. These effects would suggest that the increased fat content that results from incorporating CM into HFD altered the fat composition of the diet in favour of unsaturated fats, possibly the linoleic acids. The difference in outcome between mice fed standard diet and HFD also partly supports reports that the effects of linoleic acid on body weight and fat composition was limited to animal models of metabolic disease, while no effects were observed in healthy individuals (63, 64). Despite its effects on blood glucose or lipid profile, C. mannii was not associated with alteration of the biochemical markers of liver or kidney integrity.
Result of open field activities revealed that C. mannii was associated with a suppression of locomotion, rearing and grooming activities in all groups, irrespective of dietary composition (compared to normal control); although when compared to HFD, CM was associated with a slight increase in horizontal locomotion and rearing. The reduction in these open field activities is suggestive of a central depressant/inhibitory effect. While there is a dearth of scientific studies examining the central nervous system effects of C. mannii, central depressant effects of a given compound or drug have been attributed to their ability to downregulate or upregulate excitatory or inhibitory neurotransmitters respectively. Alterations in horizontal locomotion or grooming in the open field has been associated with changes in the activity of dopamine (DA) in a number of studies (65, 66). Methylation at the DA receptors, synaptic cleft or receptor-bound DA (67, 68), and inhibition of ligand gating to dopamine also results in a downregulation of DA receptors, resulting in locomotor retardation or a reduction of self-grooming. The stimulation of the inhibitory neurotransmitter gamma amino-butyric acid (GABA) receptors has also been associated with locomotor retardation or a central depressant response. Studies have also shown that the activation of A and B subtypes of the GABA receptors reduces grooming frequency (66, 69). The result of brain GABA levels in this study also supports the inhibitory response observed, although the rich essential amino acid content of C. mannii (4, 26) could also alter neurotransmitter balance in favour of a central inhibitory response
Spatial working memory is regulated and modulated via the influences of glutamatergic, cholinergic, or GABAergic projections linking a number of brain regions (70-72). In this study, when compared to controls that were fed SD, a decrease in Y-maze spatial working memory was observed at the highest concentration of CM; while working memory decreased at all concentrations of HFD/CM (although when compared to HFD, a significant improvement was observed with HFD/CM). Similar effects were observed in the radial arm maze in groups fed HFD/CM. Alterations in neurotransmitter levels/mechanism or antioxidant status has been associated impairment of memory (71, 72). The activation of GABAergic inputs in the median septum has been reported to impair spontaneous alternation working-memory (70, 73).In this study, an increase in brain acetylcholinesterase activity in the HFD and HFD/CM groups as well as the increase in the levels of GABA could account for the memory impairment observed in these groups.
Elevated plus maze (EPM) behaviours have been used to assess anxiety response in rodents, and it is based on the rodent’s natural aversion for elevated and open spaces. The results of the EPM test in this study revealed an anxiolytic response at all concentrations of CM irrespective of dietary composition. An anxiety-related or anxiolytic response in the EPM is usually a consequence of complex, multi-feedback mechanisms that involve numerous neurotransmitter systems including dopamine, serotonin and norepinephrine (74, 75). The ability of C. mannii to modulate any of these neurotransmitters could possibly account for the anxiolytic effect observed in this study.
In the study, C. mannii was associated with increased SOD activity in all groups when compared to the effect observed in mice fed standard diet. However, compared to HFD, a reversal of HFD induced increase in SOD activity was observed. Lipid peroxidation levels increased in groups fed HFD/CM compared to SD groups although a reversal on HFD induced increase in lipid peroxidation was observed when compared to groups fed HFD. The antioxidant effect of CM is supported by the results of the in vitro tests that demonstrated the DPPH scavenging ability and ascorbic acid content of CM; it also corroborates the results a previous study detailing the antioxidant properties of CM (76). In mice fed SD, CM was associated with increasing SOD activity, although no significant increase in lipid peroxidation levels was observed. Therefore, in the various organs that were studied, C. mannii exhibited a modulatory effect on the activity of SOD in such a way that the modulation is dependent on diet; and the overall effect can either be beneficial or detrimental.
In conclusion, this study shows that dietary C. mannii supplementation can modulate neurobehaviour, biochemical markers of liver and kidney function, lipid profile and brain levels of GABA and acetylcholinesterase activity in mice that were fed either normal or high-fat diet. In the open field, the overall effect is that of suppression of activities, while other models showed mitigation of both anxiety and memory decline. Biochemical markers of metabolism, liver/kidney function, and \lipid profile showed both beneficial and potentially-detrimental effects of C. mannii supplementation of the diet. However, improved acetylcholinesterase activity and increased GABA levels are potentially-beneficial in modulating behaviours. Therefore, while advocacy for adoption of CM as a nutritionally-versatile nutrient source continues, more research needs to be conducted in the quest for more knowledge in relation to its effects on the body.
Anthony T Olofinnade, Adejoke Y Onaolapo and Olakunle J Onaolapo conceived and designed research. Anthony T Olofinnade, Adejoke Y Onaolapo and Olakunle J Onaolapo and Azurra Stefanucci, conducted the experiments. analyzed the data and wrote the manuscript. Adriano Mollica and Olugbenga A Olowe supervised the work and ensured compliance with ethical standards. All authors read and approved the manuscript. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. All authors of this paper declare that there is no conflict of interest related to the content of this manuscript. The data is presently unavailable in the public domain because authors do not have permission to share data yet. So data would be made available only on request.
HDL-High
density lipoprotein
High-fat diet
Low density lipoprotein
Malondialdehyde
Standard diet
Superoxide dismutase
Thiobarbituric acid-reactive species
Total cholesterol
Triglyceride
