Autoantibodies to beta-1 adrenergic receptor have been reported in adult patients with dilated cardiomyopathy (DCM). Removal of these antibodies has a positive hemodynamic effect. Our aim was to investigate whether these antibodies are present in children with DCM and explore the potential hemodynamic benefit of immunoadsorption (IA). Seventeen children with DCM were tested for these antibodies. The etiology of DCM was genetic (n = 5), myocarditis (n = 4), DCM and congenital heart block (n = 3), DCM associated to maternal lupus (n = 1), DCM and Wolff Parkinson White Syndrome (n = 1), and idiopathic (n = 3). All patients evidenced ventricular dysfunction. Antibody testing was positive in 8 patients, 7 received IA. Three patients with high titers had a poor clinical outcome and needed transplantation. Two patients with low titers exhibited a full recovery of heart function. One patient with multiple myocarditis episodes was treated with immunoglobulin IgG and IA ; after 5 years this patient presented a LVEF of 40 percent. Beta-1 adrenergic receptors autoantibodies are present in children with DCM. Immunoadsorption therapy may help improve heart failure in this context.
Dilated cardiomyopathy (DCM) is a disease of the cardiac muscle characterized by progressive decline of myocardial contractility associated with dilatation of ventricles. In children, DCM is the most frequent cause of heart failure and a paramount indication for pediatric heart transplantation (1).
Over the past few decades, β1-adrenergic antibodies have been reported in sera from adult patients with DCM (2, 3). The mechanism inducing these antibodies is not well understood, but it is suspected that an infection (myocarditis) may trigger the autoimmune response (4). The β1-adrenergic receptor (β1AR) is a G-protein coupled receptor expressed predominantly on cardiomyocytes. The binding of catecholamines to this receptor activates the stimulatory G-protein Gs thereby activating adenylate cyclase, which leads to transient increases in intracellular cAMP entailing increased cardiac contractility (inotropic effect) and heart rate (chronotropic effect) (5). β1AR antibodies have an agonistic activity. They bind the β1AR of cardiomyocytes, resulting in permanent adrenergic overstimulation, apoptosis, and cell death (4, 5). Furthermore, β1AR antibodies isolated from serum samples of DCM adult patients have agonistic properties in vitro (6, 7) and active and passive immunization against the target epitopes of these autoantibodies induces DCM in rodents (8).
Presence of β1AR antibodies in DCM is associated with poorer heart function, higher frequency of arrhythmias, higher incidence of sudden death and a three-fold increased risk of cardiovascular mortality (4). Removal of β1AR antibodies by immunoadsorption (IA) in human adults with DCM has been demonstrated to have a significant hemodynamic benefit and even induce complete clinical remission, and similar effects have been obtained in animal models by systemic neutralization of β1AR antibodies (9-15). However, none of these specific therapeutic approaches has so far been tested in children. Myocarditis is the most common cause of heart failure in children with structurally normal heart. Some cases evolve to spontaneous recovery, but many others develop DCM and chronic heart failure to be cured by cardiac transplantation. Human IgG and immunosuppressors have been used for the treatment of DCM in children (16). However, the results obtained vary widely due to limited validity in therapy assessment. In myocarditis, determination of β1AR autoantibodies would be of great help in ascertaining the autoimmune etiopathogenesis of heart failure and selecting cases prone to benefit from immunological treatment specifically directed against the autoantibodies.
Our aim was to explore this possibility: To analyze whether β1AR autoantibodies are present in children with DCM, and to assess the potential hemodynamic benefit upon removal of these autoantibodies by IA.
We herein present a case series of children with DCM referred to our hospital since 2011. All children were tested for the presence of β1-adrenergic receptor autoantibodies. Positive cases were treated with IA prior informed consent.
The concentration of β-1 adrenergic receptor autoantibodies was measured in serum samples collected locally and shipped to Celltrend Facilities (Lukenwalde, Berlin). An ELISA method was used for the determination, which uses native membranes of β1AR-overexpressing cells as an antigenic target. Titers above 15 U/ml were considered positive.
Autoantibody titers were quantified before and after each IA cycle and subsequently controlled monthly. The procedure was repeated until titers remained negative.
Extracorporeal IA was conducted by pediatric nephrology staff in three to four 2-hour sessions or cycles (1 session a day) in the intensive care unit or the nephrology outpatient clinic, depending on the child clinical status. A hemofiltration catheter was inserted.
The immunoadsorption of immunoglobulins was done by using the TheraSorbTM LIFE 18 Apheresis Unit (Miltenyi Biotec GmbH, Germany) and the TheraSorbTM Ig flex adsorbers (one adsorber pair; Miltenyi Biotec GmbH, Germany) which are consecutively regenerated after each treatment (intended for up to 10 uses). For the specific elimination of immunoglobulins, an ovine-prepared anti-human immunoglobulin polyclonal antibody covalently bound to a solid matrix of Sepharose CL-4B was used. This matrix is suspended in 0.9.% NaCl and the extracorporeal volume is 45 ml.
This technology allows the specific removal of any class of immunoglobulin (IgG, IgM, IgE, IgA) as well as any subclass of IgG (IgG1-4). The apheresis unit TheraSorbTM LIFE 18 is an integrated plasma therapy platform composed by 1) TheraSorbTM LIFE 18 Monitor, 2) a pair of immunoadsorbent columns, 3) tubing set, 4) plasma disk separator, and 5) rinsing, washing and storage solutions.
Plasma loading and processing are performed automatically by the LIFE 18 apheresis system. The main steps are summarized below. At the beginning of each session the patient blood was extracted by the venous access and, adequately anticoagulated, was directed to the separator disk of the apheresis unit, which separates the plasma from its cellular components. The plasma was then directed to the column which captured human immunoglobulins and, upon re-binding to its cellular components, the blood was reinfused to the patient. The Life 18 monitor alternately charges the two columns of plasma so that while one is filling with plasma, the other is regenerating.
In each cycle, each column or adsorber was loaded with 150 ml of plasma and, after a bolus of 6 ml of physiological saline, washed with 40 ml of the same solution, regenerated with 100 ml of acid glycine (pH 2.8.) and, due to the pH decrease, human immunoglobulins were released from the sepharose matrix. The binding sites were again free for correct adsorption in future cycles. To increase the pH, the column was neutralized with 115 ml of PBS and finally irrigated with 100 ml of physiological saline, leaving the column waiting for the next cycle. As each cycle purifies 150 ml of plasma, the number of cycles of the treatment was calculated depending on the plasma volume to be purified. The extracorporeal volume of the system is only 100 ml, making it an ideal technique in pediatrics. In children younger than 10 kg the extracorporeal circuit was primed with 5% albumin. We used a blood flow around 3-5 ml/kg/minute. Anticoagulation was done with an initial iv bolus of fractionated heparin (1 mg/kg BW) and a regional infusion of 3% citrate:blood flow (1:28-30, v/v) (ACDA, Fresenius Kabi). ACT and free Ca2+ levels were repeatedly monitored during the procedure, with the purpose to maintain a concentration of 0.2.-0.3. mMol/L free Ca2+ at the absorbed outflow and 1-1.3. mMol/L in peripheral blood. An adjusted suspension of 10% calcium gluconate i.v. (blood flow (ml/min) x K; K= 0.2.5; 0.2.5 ml/kg BW/h) was regularly infused into the patient since the beginning of the apheresis in order to avoid hypocalcemia. Each immunoadsorption session consisted of 2 complete plasma volumes.
At the end of the procedure, each absorber is finally preserved with at least 500 mL PBS-Azide buffer (pH = 1.6.). After this step, adsorbers can be removed and stored at 2-3 ºC. Each pair of adsorbers has been intended to be used up to 10 times for the same patient, with an expiration time of use of 60 days since its first use. After the last session an infusion of immunoglobulin was administrated (Dose 0.5. to 1.0. g/kg).
All patients had an echocardiography study performed at baseline and after IA cycles. LVEF was estimated using the Simpson’s method. In addition to changes in LVEF, the hemodynamic benefit was assessed by changes in the functional class by the Ross scale or the NYHA classification and measuring brain natriuretic peptide (BNP) before and after each cycle.
Seventeen children were tested whose DCM etiology included: genetic causes (n = 5), myocarditis (n = 4), congenital heart block (n = 3), maternal lupus (SLE) (n = 1), Wolff Parkinson White syndrome (n = 1), or was idiopathic (n = 3). Table 1 gives a descriptive summary of all 17 cases. Antibody testing was positive in 8 patients (47%): 2 patients with DCM and heart block, 2 patients with idiopathic DCM, and 4 patients with myocarditis. All genetic-based cases tested negative. All children evidenced moderate-to-severe dysfunction with a left ventricle ejection fraction (LVEF) lower than 50% and NYHA functional class III-IV.
| Pt # | Age | Etiology | LVEF | NYHA | β-1 ARA (U/ml) | IA (cycles) | Outcome |
|---|---|---|---|---|---|---|---|
| 7 mo. | Maternal SLE + heart block | 30% | IV | 260.00 | 1 | Transplant | |
| 1.3y | Maternal SLE + heart block | 30% | IV | 170.00 | +4 | LVEF 45%. Died (sepsis) | |
| 1.5y | Idiopathic | 40% | III | 111.00 | 1 | Transplant | |
| 9 mo. | Idiopathic | 26% | IV | 91.00 | 3 | Transplant | |
| 1y | Myocarditis (CMV) | 30% | IV | 29.00 | 2 | Recovery LVEF 56% | |
| 2 y | Myocarditis (Kawasaki) | 40% | IV | 27.00 | 1 | Recovery LVEF 60% | |
| 5 y | Myocarditis (Parvovirus) | 40% | IV | 16.40 | 4 | LVEF 45% NYHA II | |
| 13 day | Myocarditis (Enterovirus) | 25% | IV | 16.20 | No | Died | |
| 13 y | Genetic | 30% | IV | 4.73 | No | Transplant | |
| 8 y | Genetic | 25% | IV | 7.48 | No | Transplant | |
| 13y | Genetic | 30% | IV | 6.09 | No | Transplant | |
| 12 y | Genetic | 25% | IV | 1.88 | No | Transplant | |
| 1.2y | MaternalSjögren +Heart block | 34% | IV | 2.50 | No | Listing for Trasplant | |
| 3 mo. | Genetic | 30% | IV | 5.90 | No | Transplant | |
| 1 day | Maternal LSE | 40% | IV | 3.50 | No | Alive | |
| 4 days | Idiopathic | 20% | IV | 4.10 | No | Died | |
| 5 mo. | MCD +WPW | 50% | IV | 5,60 | No | Recovery LVEF 55% | |
| Abbreviations: Pt, patient; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; β-1 ARA, β-1 adrenergic receptor autoantibodies; IA, immunoadsorption; mo., months; y, years DCM, dilated cardiomyopathy; SLE, systemic lupus erythematosus WPW : Wolff Parkinson White Syndrome | |||||||
The four cases (1 through 4) with the highest titers of β1AR-autoantibodies had severe heart failure. The IA technique was well tolerated and effective in lowering β1AR-autoantibodies, the titers of which were tested negative immediately after IA in all cases. However, patients #1 and 4 experienced a strong rebound of titers and worsening of heart function and had to be heart transplanted.
Patient #3 suffered a septic episode catheter-related after an IA cycle; antibody titers went up again in parallel to a clinical worsening, and this case ended up in transplantation as well.
Case #2 was a child critically ill with DCM and heart block associated with maternal lupus. She had a very high titer of β1AR-autoantibodies before IA, but subsequently, titers remained below the cut-off for a month (See Figure 1). Left ventricular function improved notably after the second IA cycle (from NYHA IV to NYHA II and from LVEF 30% to LVEF 45%) and remained stable during several subsequent cycles of IA. Unfortunately, the patient died two years later due to sudden death associated with an infection episode with a high fever. The necropsy could not be done.
 Figure 1.
Figure 1.Evolution of β1 adrenergic antibody titers in case #2. β1AR-autoantibody were measured after each myocarditis episode by an ELISA using native cell membranes of β1AR-overexpressing cells as antigenic target . Black arrows correspond to immunoadsorption cycles.
Two patients with low positive titers and DCM due to myocarditis (#5 and 6) improved after a single cycle of IA and titers remained negative. Both children exhibited a complete and stable recovery of heart function.
Case #7 was a child with DCM due to parvovirus myocarditis. During follow up, the patient suffered multiple myocarditis episodes. We noted that titers of beta-1 adrenergic antibodies increased after each episode (Figure 2). She received several IA cycles and immunoglobulin monthly and thereupon presented negative β1AR-autoantibody titers for a year. After a 5 years of follow-up, she imposed with DCM and moderate left ventricular dysfunction (LVEF 40%, NYHA II), but did not exhibit any further myocarditis episodes.
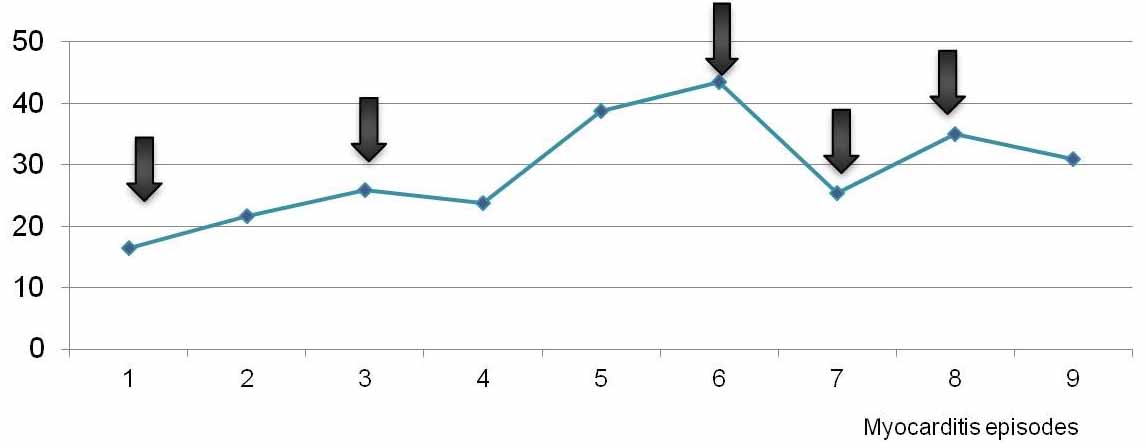 Figure 2.
Figure 2.Case #7 experienced several episodes of myocarditis in which titers of β1 adrenergic antibodies were measured. β1AR-autoantibody were measured after each myocarditis episode by an ELISA using native cell membranes of β1AR-overexpressing cells as antigenic target . Arrows represent the immunoadsorption cycles.
In all patients, BNP was reduced following IA. The mean reduction was 67% after the first cycle (See Figure 3).
 Figure 3.
Figure 3.Decrease of BNP levels with immunoadsorption cycles.
In this report, we have demonstrated the presence of β1AR-autoantibodies in children with DCM (as measured by the ELISA procedure offered by CellTrend, Luckenwalde), and we provide evidence for the potential therapeutic value of IA in β1AR-autoantibody positive cases.
The mechanism for the production of these antibodies is not well understood. It is suspected that the cause may be a cardiac viral infection (myocarditis) which triggers an autoimmune response (4, 8). Case #7 strongly reinforces this hypothesis, as it clearly indicates that the incidence and titers of β1AR-autoantibodies are correlated to episodes of parvovirus-induced myocarditis as well as the clinical symptoms of left ventricular dysfunction.
The above hypothesis is inversely supported by the observed complete absence of β1AR-autoantibody in all cases genetic DCM. Our data thus provide strong support to the notion that a subset of DCM is a humoral auto-immune disease triggered by viral cardiomyositis and mediated by β1AR-autoantibodies thereby induced.
Of note, highest titers and rapid disease rebound following IA were seen in cases with poorer outcome, and lowest titers without rebound were seen in children with a recovery of heart function. Thus, β-1 adrenergic receptor antibodies titers may be considered as a prognostic biomarker of heart failure and therapy response in this context.
IA technique removes immunoglobulins effectively, especially IgG. Three sessions were usually enough to remove up to 65% immunoglobulins IgG. We performed 3- 4 sessions (1 cycle) to achieve negativization of titers. The IA is a very powerful therapeutic tool which has been used with several autoimmune diseases, such as dilated cardiomyopathy (10, 11, 17) , acquired hemophilia, systemic lupus erythematosus, myasthenia gravis, multiple sclerosis, and to remove autoantibodies of highly hyperimmunized recipients prior to a solid organ transplantation or immediately after, in the management of an acute humoral. In case #2, we measured IgG in all cycles and confirmed the expected efficacy (865 mg/dl in the first cycle, 491 mg/dl in the second, and 311 mg/dl in the third one).
The IA technique was done by nephrology staff. It is a safe procedure because low blood and plasma flows are used producing a minimal hemodynamic instability even in the most severe cases. The procedure could be performed in all cases where it was indicated, except for a patient (case #8) with myocarditis due to enterovirus with severe heart failure who required extracorporeal membrane oxygenation support. Each patient had an exclusive chip card where all information related to the procedure was stored.
In summary, this report demonstrates the presence of β1AR-autoantibody in the serum of children with DCM but not in all cases. We believe that autoantibodies determination is important because it will allow us to recognize autoimmune mechanisms of heart failure.
Our next step will be to establish reference values of β1-adrenoreceptor autoantibodies in healthy children in order to obtain a cut off value. With this information, we should be able to estimate the real prevalence of these autoantibodies in children with DCM and then apply immunological therapies (IA and others) concomitant to conventional treatment to improve or even reverse heart failure in children with DCM.
