Academic Editors: David H. Barad and Michael H. Dahan
Background: Information about the prevalence of fallopian tube
pathology in the early stages of endometriosis is scarce. The aim of our study
was to examine the association between genital endometriosis and the lengths of
fallopian tubes in infertile women. Settings and Design: retrospective cohort
study. Methods: We studied 651 infertile patients in the 20–40 year age
group, who visited a Reproductive Clinic for treatment between 2012–2018. After
laparoscopy, endometriosis (detected by histomorphology) was indicated in 472
cases and absent in 179 cases. The length of the fallopian tubes was estimated in
193 primary infertile women with endometriosis. We excluded patients from the
study who had surgical intervention on their tubes. Appearance of tubes and
fimbriae was assessed in 177 patients without endometriosis and in 461 patients
with endometriosis. Results: The proportion of women with shorter (
Endometriosis is a condition in which endometrial tissue, which normally lines the uterus, develops outside of the uterine cavity in abnormal locations such as the ovaries, fallopian tubes, and the abdominal cavity [1].
Classical studies suggested that 25% to 50% of infertile women have endometriosis. According to Nicolaus K et al. [2], its incidence in infertile women reaches 67% and that 30% to 50% of women with endometriosis are infertile [3].
Peritubal and peri-ovarian adhesions are considered the main mechanisms by which advanced endometriosis causes infertility. The mechanisms by which adhesion formation in advanced endometriosis reduces fecundity rate include a reduced ovum pick-up mechanism, luteinized un-ruptured follicle syndrome, poor follicular growth, and tubal occlusion [4].
Information about the prevalence of fimbrial pathology of fallopian tubes in early stages of endometriosis is scarce. Peritubal and peri ovarian adhesions are considered the main mechanisms of infertility caused by progressive endometriosis [5].
In addition, endometriosis without adhesions may contribute to reduced fertility potential [6]. On the other hand, the mechanisms by which early stages of endometriosis interfere with fertility are not fully understood.
The reference standard for diagnosing endometriosis is laparoscopy, preferably including histological verification by biopsy of any suspected lesion [7].
The aim of our study was to determine the correlation between genital endometriosis and fallopian tube pathologies.
A retrospective cohort study was conducted. Patients were recruited between June
2012 and December 2018. A total of 651 infertile women underwent laparoscopy in
our clinic. All the women met the following criteria: age between 20 and 40
years; infertile for
 Fig. 1.
Fig. 1.Measurement of the length of the left and right fallopian tube. (a) Ampullary part. (b) The end of fimbriae.
 Fig. 2.
Fig. 2.Fimbriae.
Laparoscopy was performed on any day of the menstrual cycle under general anaesthesia. Endometriosis was diagnosed and staged according to the revised classification of the ASRM. In this classification, endometriosis is divided into four stages according to the number of lesion and depth of infiltration: minimal (Stage I), mild (Stage II), moderate (Stage III) and severe (Stage IV) [9].
After laparoscopy, a total of 461 infertile cases (Group I) were diagnosed with endometriosis by histomorphology. They were assigned to stage I or II groups according to ASRM classification. Laparoscopic treatment included excision of ovarian endometriosis lesions, lysis of adhesions, endo coagulation of pelvic endometriosis lesions with controlled heating (100 degrees C) and lavaging of the peritoneal cavity. The duration of follow-up after laparoscopic surgery was censored at 36 weeks.
Group 2 consisted of 177 infertile patients who had no endometriosis by laparoscopy and histomorphology.
From our study sample of 651 women, we separately distinguished 193 patients with primary infertility (with or without endometriosis) and evaluated the length of their fallopian tubes. The aim of the study was to compare the length of tubes in patients with or without endometriosis and determine the association between tube length and endometriosis.
Hysterosalpingographia was done in the first half of menstrual cycle, on protected sexual intercourse. Under aseptic conditions, a HSG 5-French balloon tipped catheter was inserted through the cervix into the uterine lumen. Sterile contrast material was utilized to distend the uterine lumen and fallopian tubes. Images were obtained after 6 minutes [10, 11, 12].
We refined our protocol for hysterosalpingographya and for assessing normal and abnormal fallopian tube patency [10].
We measured the fallopian tubes during laparoscopy using a device we developed. A sterile flexible plastic thread (polyethylene) or suture thread was introduced from the 5 mm trocar into the abdominal cavity. One end of the thread was positioned to the cornua of the tube, care was taken that the thread was lying all along the tube until reaching the end of the fimbria. The thread was then cut at this point, removed from the abdominal cavity and measured in centimeter. The length of fallopian tubes is the maximum and minimum lengths we have obtained by laparoscopic measurements.
Fimbrial pathology was identified in all involved patients, especially fimbrial adhesives and fimbrial phimosis. Fimbrial phimosis describes an actual narrowing of the fimbriated end. If fimbrial pathology was found, it was classified as unilateral or bilateral.
We have calculated the rate of big (Morgani cyst) and small (appendix vesiculosa) paratubal cysts in both groups of patients. See Table 1.
| Factors | Without endometriosis | At endometriosis | Total | ||
| (n = 177) | (n = 461) | (n = 638) | |||
| Appearance of the right fallopian tube n (%) | |||||
| Normal | 130 (73.4%) | 266 (57.7%) | 396 (62.1%) | ||
| Adhesions | 4 (2.3%) | 21 (4.6%) | 25 (3.9%) | 0.181 | |
| Small paratubal cyst | 37 (20.9%) | 64 (13.9%) | 101 (15.8%) | 0.030 | |
| Morgani cyst | 6 (3.4%) | 48 (10.4%) | 54 (8.5%) | 0.004 | |
| Endometriotic lesions | 0 (0.0%) | 62 (13.4%) | 62 (9.7%) | ||
| Appearance of the fimbriae of right fallopian tube n (%) | |||||
| Normal | 164 (92.7%) | 397 (86.1%) | 561 (87.9%) | 0.023 | |
| Adhesion | 13 (7.3%) | 64 (13.9%) | 77 (12.1%) | 0.023 | |
| Appearance of the left fallopian tube n (%) | |||||
| Normal | 140 (79.1%) | 310 (67.2%) | 450 (70.5%) | 0.003 | |
| Adhesions | 4 (2.3%) | 57 (12.4%) | 61 (9.6%) | ||
| Small paratubal cyst | 25 (14.1%) | 46 (10.0%) | 71(11.1%) | 0.136 | |
| Morgani cyst | 8 (4.5%) | 35 (7.6%) | 43 (6.7%) | 0.166 | |
| Endometriotic lesions | 0 (0.0%) | 14 (3.0%) | 14 (2.2%) | 0.019 | |
| Appearance of fimbriae of the left fallopian tube n (%) | |||||
| Normal | 173 (97.7%) | 418 (90.7%) | 591 (92.6%) | 0.003 | |
| Adhesion | 4 (2.3%) | 43 (9.3%) | 47 (7.4%) | 0.003 | |
| p | |||||
We have no data about the ectopic pregnancy rate after laparoscopy in the study. We think it is an interesting issue to consider in the future. Informed consent was obtained in advance from all patients participating in the study.The study was approved by the Ethics Committee of the clinic.
Patients with infertility duration of more than 2 years with or without endometriosis symptoms.
Genetic anomalies of the woman or her partner, male factor infertility, a history of pelvic or abdominal surgery for tubal ligation, chlamydia or gonorrhea infection; pelvic inflammatory disease (PID).
Medical history, clinical-biochemical laboratory, morphological laboratory, ultrasound examinations, hysterosalpingography.
Statistical analysis was performed using SPSS for Windows, version 23 (SPSS Inc,
Chicago, IL, USA). Correlation analysis between categorical variables was
performed by Spearman correlation analysis, p value
Among laprascopically examined patients, endometriosis was detected by histomorphology in 472 cases and was absent in 179 cases.
Tubal patency prior to laparoscopy is shown in Fig. 3.
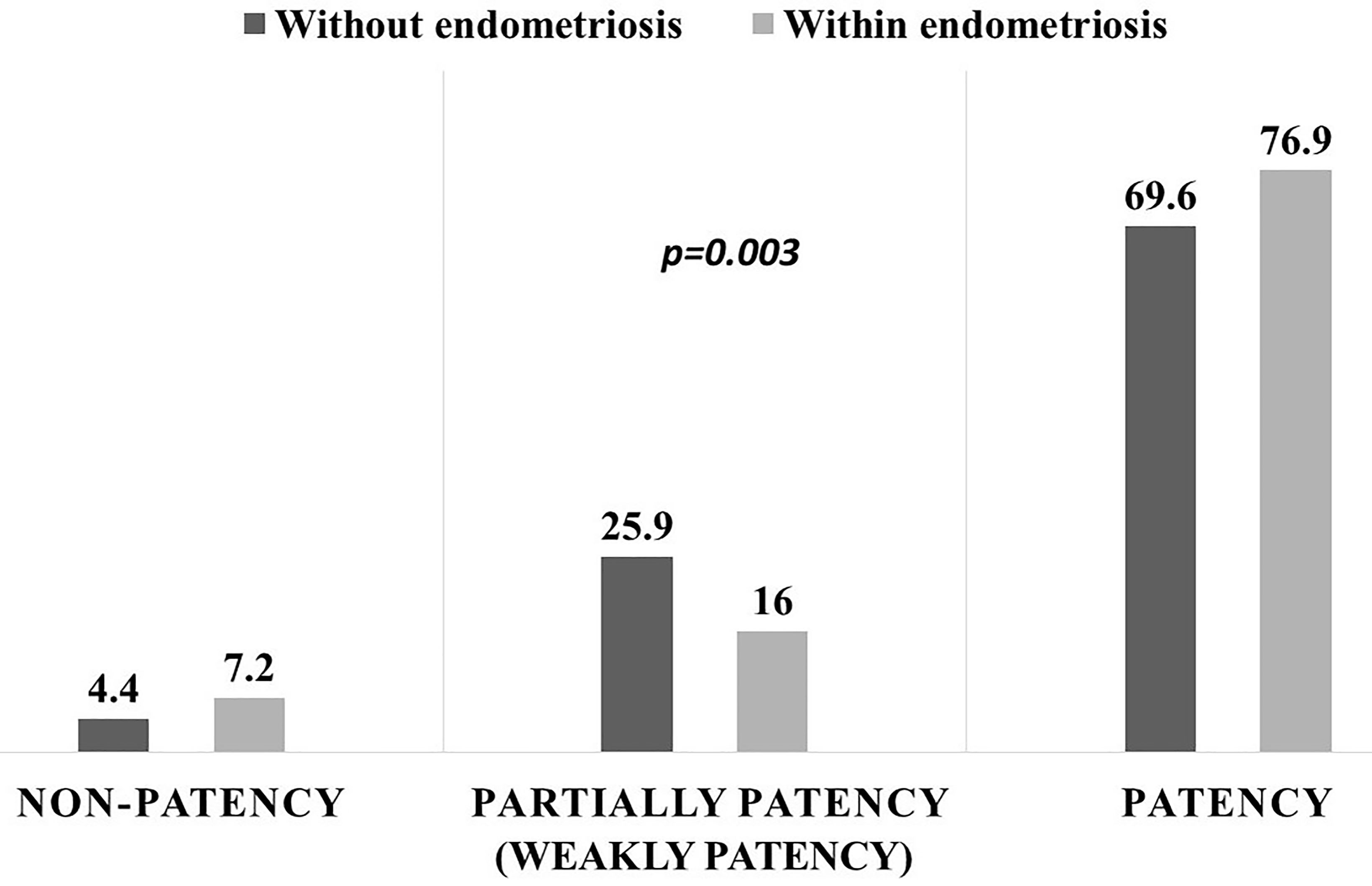 Fig. 3.
Fig. 3.Tubal patency prior to laparoscopy defined by hysterosalpingography.
The rate of non-patent tubes in the group of patients with endometriosis was
7.2% and in the group without endometriosis 4.4%. Weakly (partial) patent tubes
was 25.9% in the group with endometriosis and 16% without it.
After laparoscopy it was found that in 96.8% of patients with endometriosis have completely patent tubes
The length of the fallopian tubes was estimated in 193 cases. Table 2 shows the length of fallopian tubes measured during laparoscopy.
| Factors | Without endometriosis n (%) | With endometriosis n (%) | Total n (%) | p | |
| N = 57 | N = 136 | N = 193 | |||
| Length of the right fallopian tube (cm) | |||||
| 5 (8.8) | 87 (64.0) | 92 (47.7) | |||
| 8–12 | 21 (36.8) | 46 (33.8) | 67 (34.7) | 0.183 | |
| 31 (54.4) | 3 (2.2) | 34 (17.6) | |||
| Length of the left fallopian tube (cm) | |||||
| 7 (12.3) | 70 (51.5) | 77 (39.9) | |||
| 8–12 | 16 (28.1) | 57 (41.9) | 73 (37.8) | 0.540 | |
| 34 (59.6) | 9 (6.6) | 43 (22.3) | |||
| Total | 57 (29.1) | 136 (70.9) | 193 (100.0) | ||
| p | |||||
The proportion of short (
Laparoscopy also showed significant differences between the groups in the appearance of fallopian tubes and fimbriae of fallopian tubes, assessed during the laparoscopy (Table 1). We excluded patients from the study who had surgical intervention on their tubes.
Appearance of tubes and fimbriae was assessed in 177 patients without endometriosis and in 461 patients with endometriosis.
The proportion of women with normal appearance of fallopian tubes and fimbriae was significantly lower in women with endometriosis. The proportion of women with adhesions and Morgani cysts was higher in the group of patients with endometriosis. The occurrence of small paratubal cysts (appendix vesiculoza) was greater in the group of patients without endometriosis.
Correlation analysis showed significant positive correlations of endometriosis
with: a short (
Endometriosis is a common, oestrogen driven chronic gynaecological condition, where endometrium-like epithelial and stromal cells exist in ectopic sites beyond their native location; the internal lining of the uterine cavity. Approximately 10% of reproductive age women are affected by endometriosis, which equates to around 190 million women worldwide [13, 14]. It is most commonly associated with chronic pelvic pain (CPP) and accounts for up to 70% of such diagnoses [14, 15]. Other associated symptoms include pain during menstrual periods (dysmenorrhoea) and/or sexual intercourse (dyspareunia), intermenstrual bleeding and infertility [15]. The most common sites for endometriotic lesions to establish are the ovaries and pelvic peritoneum, yet endometriotic lesions are also found in other sites such as the abdominal wall, fallopian tubes (FT), bowels, bladder, cervix and vagina [14]. Several studies confirmed that infertile women are 6 to 8 times more likely to have endometriosis than fertile women [7].
Fallopian tubes (FT) are implicated in all endometriosis-associated symptomatology and clinical consequences. They may contribute to the origin of endometrial tissue, determine the sites of formation of ectopic lesions and serve as conduits for the spread of proinflammatory media [16, 17].
The epithelial cells of the fallopian tube exist as a continuum of the endometrium, the mucosa lining the uterine cavity and both tissues share the same embryological origin [17].
The shared embryological origin and some similar phenotypical and functional features between the FT and endometriosis compelled us to examine FT involvement in the genesis, pathophysiology and clinical consequences of endometriosis. There are many interesting findings suggesting that both endometrium and tubal mucosa may contribute to the origin of the disease and that presence of endometrial lesions may cause tubal dysfunction [16].
The tortuous course of the intramural portion of the uterine tubes constitutes a normal anatomical finding. It controls the retrograde flow of blood during menstruation and reduces the possibility of developing endometriosis. Straight or curved intramural portions represent an anatomical abnormality that may predispose women to endometriosis [18, 19]. Endometriosis was more frequent in women with straight courses and was infrequent in women with tortuous ones [17].
The presence of adhesive disease that may entrap the fimbria in infertile patients with moderate and severe endometriosis is clearly understood. It represents part of fallopian tube involvement in adhesion formation after treatment of active pelvic endometriosis. However, little is known about the prevalence of fimbrial pathology in the early stages of endometriosis.
The most widely accepted theory of endometriosis suggests that it occurs during
retrograde menstruation, when fragments of menstrual endometrial tissue are
refluxed into the pelvic cavity through fallopian tubes and stored for
development of ectopic endometrial lesions [20, 21]. There are data on the
correlation between the length of the fallopian tubes and infertility [22]. We
can assume that retrograde menstruation is facilitated by the shorter length of
fallopian tubes, which is confirmed by our study showing that among infertile
women with endometriosis, the proportion of women with short fallopian tubes
(length
It has been suggested that women with endometriosis exhibit diminished physiological utero tubal transport capacity compared to the control subjects [23], which may be due to tubal pathologies. Since fallopian tubes and uterine tissues have the same embryological origin [16, 24], that may explain the link between endometriosis and the unusual appearance of fallopian tubes. In addition to endometriosis located in the fallopian tube, tubular abnormalities can also occur with the diagnosis of pelvic endometriosis elsewhere [25]. In our study, endometriosis showed a negative correlation with the normal appearance of fallopian tubes. The rate of adhesions and endometrial sites in the group of patients with endometriosis was significantly higher.
Causes of tubal dysfunction in endometriosis could be tube blockage, adhesion formation, or hydrosalpinx. All of these conditions of the disease are caused by inflammatory processes. They can co-exist, and are often interrelated, making it difficult to distinguish the exact contribution of each cause in the complete functional disorder of fallopian tubes. About 30% of women with endometriosis show any type of tubal pathology [26, 27].
The prevalence of fimbrial pathology was significantly higher in infertile
patients with early stages of endometriosis (50.2%) compared with infertile
patients with no endometriosis (17.8%, p
This should explore the significance of the results of the work, not repeat them. A combined Results and Discussion section is often appropriate. Avoid extensive citations and discussion of published literature.
Infertile women with minimal or mild endometriosis have been shown to have anatomical and structural changes in the fallopian tubes and fimbriae compared to those without endometriosis. The presence of genital endometriosis (I;II stage) maybe associated with shorter fallopian tubes.
LI, TV designed the research study, LI performed the research, LI, RG, LT analyzed the data, IT provided help and advice on statistical analysis, LI, AG, TV wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
All participants in the study signed an informed consent form.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
