†These authors contributed equally.
Academic Editors: Michael H. Dahan and Shigeki Matsubara
Background: The success of urogynecology synthetic grafts depends on
adequate tissue reinforcement. This experimental animal study aimed to determine
the abdominal wall reinforcement achieved by different urogynecology synthetic
grafts, including the influence of inflammatory cells, collagen deposits, and
tissue-induced oxidative stress. Methods: Electron microscopic analysis
of six different grafts, all with Polypropylene as their major component, was
performed to determine the primary mesh characteristics. Full-thickness abdominal
wall defects were repaired using monofilament, multifilament, and coated grafts
in male Wistar rats. After six weeks, the animals were sacrificed and the
inflammatory response, collagen deposition, and oxidative stress levels were
quantified. Using the digital acquisition system (Hottinger Baldwin Messetechnik
(HBM) “Catman Easy”, Darmstadt, Germany), mechanical testing of the native grafts
and of the reinforced abdominal wall was conducted and measured in a controlled
environment. Multivariate analysis was performed to determine the predictive
value of inflammatory cell numbers, collagen amount, oxidative stress, and native
graft strength on the final abdominal wall reinforcement. Results: The
inflammatory response was significantly more prominent with the multifilament
polypropylene compared to the low-weight monofilament polypropylene (p
Synthetic grafts have demonstrated superior durability and long-term success over biomaterials for treatment of stress urinary incontinence [1]. Biocompatibility has been established as the most crucial factor for both short- and long-term success [2, 3]. Quantifying the biocompatibility and establishing its statistical relationship with abdominal wall reinforcement can bring further understanding of polypropylene meshes. Graft variables, such as mesh thickness, mesh weight, pore surface, and the oxidative stress levels induced, may also define the quality of a reinforcement [4]. An interesting factor that is emerging as possibly influencing the overall success of such grafts may be the cell oxidative stress induced.
This experimental animal study aims to evaluate, through multivariate analysis, multiple graft and biocompatibility factors to identify their influence on the quality of the reinforcement and on the overall success of the procedure.
Six different types of polypropylene graft were used in the experiments: high
weight polypropylene (HWPP; Prolene mesh, monofilament, Ethicon, UK, 76
g/m
For each type of polypropylene graft, three specimens, standardized to 40
A total of 144 male Wister rats, each weighing 200/250 g, were divided into six
groups (24 animals in each group), each group being assigned for use with one of
the grafts. All experimental procedures involving the animals were conducted in
compliance with the European Council Directive (EU directive of 2021; 2010/63/EU)
and the Guide for the Care and Use of Laboratory animals (10th edition, National
Academy Press). The Ethics Committee for Animal Experimentation of the Faculty of
Medicine, University of Nis, approved the experimental study. The animals were
anesthetized with 0.3 mL 10% Ketamidor (Richter, Austria) injected
subcutaneously, dosed in relation to body mass. Monofilament, multifilament, and
coated polypropylene grafts were used for the primary repair of a full thickness
abdominal wall defect (20
Tissue samples were stored in a 10% buffered formalin solution and then were
fixed and dehydrated in ethanol solutions of increasing concentration (50%,
70%, 90%, and 100%). Upon dehydration, samples were fixed in paraffin blocks
(boiling point: 58 °C) and then sliced into 3–5
 Fig. 1.
Fig. 1.Polarized microscopy of H&E samples used for inflammatory cell quantification- polypropylene graft position and inflammatory cells in the near proximity of the graft verified in all samples.
Precise collagen quantification was performed from tissue stripped directly from the surface of the grafts, as described by da Silva et al. [4]. The alkaline hydrolysis of collagen in fresh samples was performed as described to obtain a sensitive hydroxyproline assay of hydroxylates. Colorimetric determination (spectrophotometer SP-22, Bio Spectro, Curitiba, Brazil with 1 cm optical glass cuvettes) was performed for hydroxyproline for alkaline hydrolysates in 1/100 dilution. A 50% w/v stock solution of NaOH (Vetec Brazil, CAT No 101) was used to prepare the samples. All samples underwent 40 minutes of hydrolysis, and PH correction was conducted identically on all samples, using a PH meter (model HI3222, Hanna, Instruments, USA).
For the oxidative stress analysis, tissue samples removed from the graft were homogenized and then spun at 1500 rpm for 10 minutes. Protein levels in the tissue homogenates were then quantified using Lowrey’s method (1951). Malondialdehyde (MDA) levels, as the final product of lipid peroxidation, were quantified (nmol/mg protein) in a 10% homogenate using the thiobarbituric acid (TBA) method (Okhava, 1979). Sample preparation and analysis were conducted at the Laboratory for Medical Biochemistry at the Faculty of Medicine, University of Nis.
A linear correlation test, the Kruskal-Wallis analysis of variance (ANOVA),
followed by the Mann-Whitney U-test, was used for the pair analysis. Bonferroni
corrections were applied for paired comparison (Statistical package SPSS 11,
Chicago, IL, USA was used for all analyses). p values
Electronic microscopic analysis of the dry meshes indicated significant
differences in pore size for the samples tested (Table 1). The most prominent
pores were recorded in MPPG, and the least prominent in CPP, MPP, and TPP
(p
| Graft type | Pore surface. | Filament thickness. | Graft thickness. | |||
| mm |
SD | mm | SD | mm | SD | |
| HWPP | 0.570 | 0.020 | 0.086 | 0.009 | 0.650 | 0.010 |
| LWPP | 0.490 | 0.010 | 0.068 | 0.013 | 0.430 | 0.020 |
| MPP | 0.190 | 0.010 | 0.259 | 0.002 | 0.440 | 0.010 |
| MPPG | 1.060 | 0.080 | 0.190 | 0.005 | 0.340 | 0.020 |
| CPP | 0.080 | 0.030 | 0.077 | 0.003 | 0.630 | 0.020 |
| TPP | 0.460 | 0.030 | 0.048 | 0.007 | 0.280 | 0.010 |
| HWPP, high weighted polypropylene; LWPP, low weight polypropylene; MPP, multifilament polypropylene; MPPG, Multifilament polypropylene with polyglactin; CPP, collagen coated polypropylene; TPP, tetanized polypropylene. | ||||||
The samples presented with significant differences when the native mesh samples
were tested for minimal disintegration load (Fig. 2). Almost identical minimal
disintegration loads were detected for HWPP and MPP. A comparable graft strength
was presented by the CPP samples, while LWPP (p
 Fig. 2.
Fig. 2.Experimental results for native (dry) mesh-uniaxial tension test.
The abdominal wall reinforcement testing is presented in Fig. 3. While the TPP
was weakest on the native graft testing, it presented as the strongest abdominal
wall reinforcement, reaching a 15.8 N minimal disintegration load. A
statistically significant difference was reached for TPP vs. HWPP (p
 Fig. 3.
Fig. 3.Uniaxial tension test of abdominal wall explants after six weeks.
Oxidative stress levels relative to graft weight are presented in Fig. 4.
Overall, the lowest expression of oxidative stress levels was recorded for the
TPP group. When the MPP and MPPG groups were compared as multifilament groups,
there was no statistically significant difference between them (p =
0.56). No statistically significant difference was shown in oxidative stress
levels when comparing the TPP and LWPP groups (p = 0.21) or the TPP and
MPPG groups (p = 0.32). A significant difference in oxidative stress
levels was evident when comparing HWPP to TPP (p
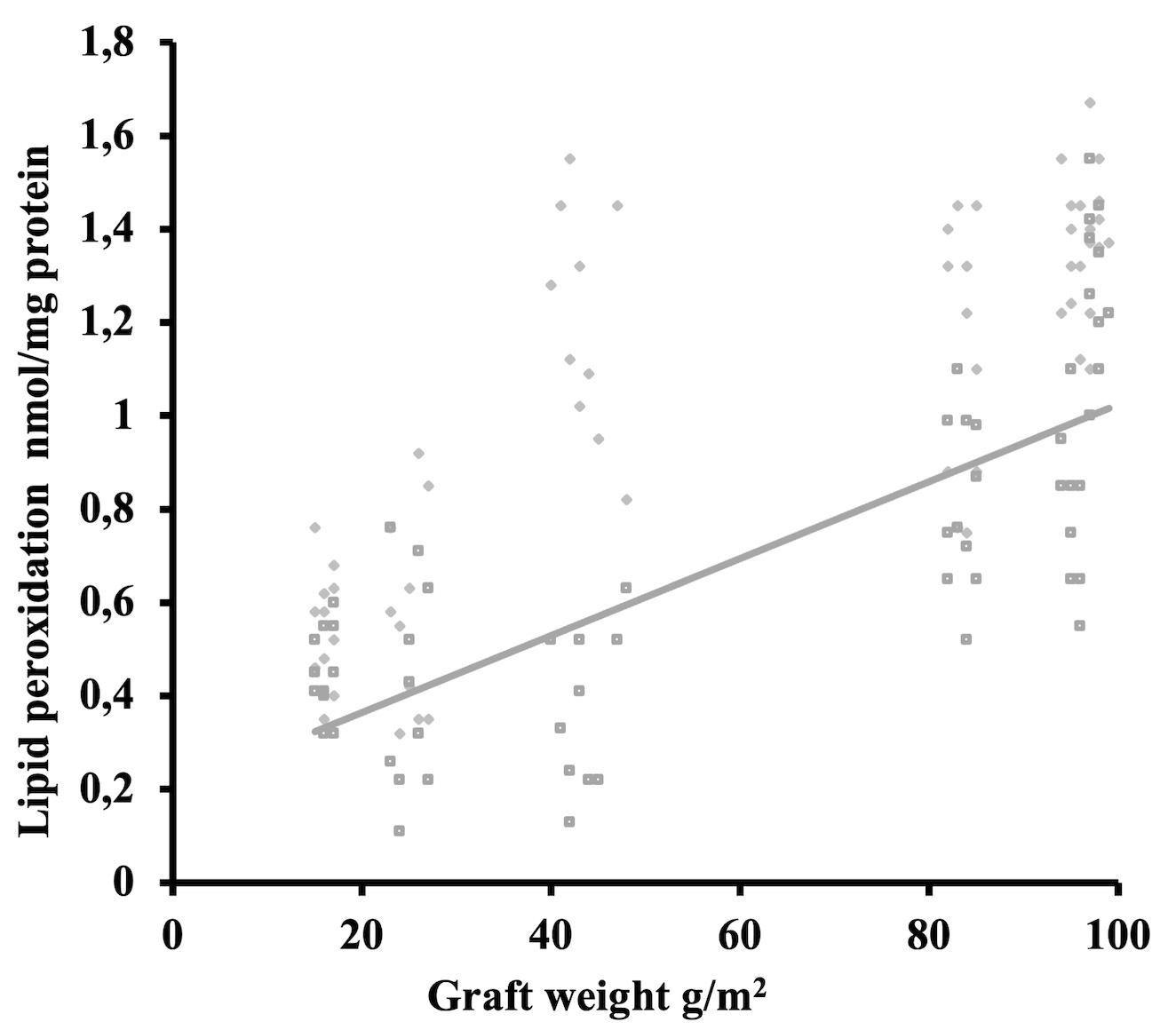 Fig. 4.
Fig. 4.Induced oxidative stress level results in accordance to graft weight (line presenting Pearson’s correlation).
The results of the inflammatory cell quantification are presented in Fig. 5.
Significant differences are noticeable among the tested grafts: MPP recorded the
most pronounced inflammatory reaction in comparison to HWPP, LWPP, CPP, and TPP
(p
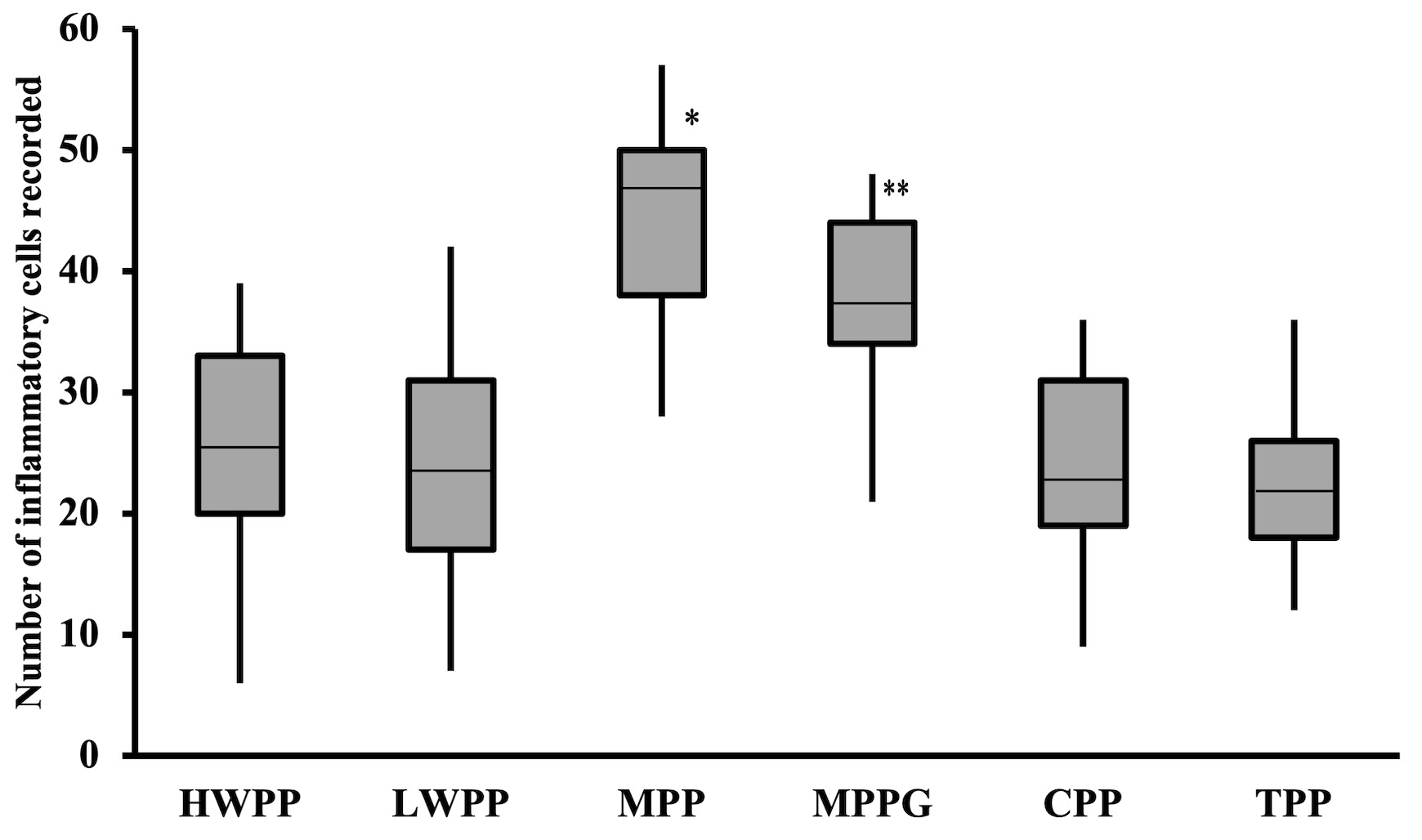 Fig. 5.
Fig. 5.Number of inflammatory cells recorded per high power field after
six weeks (median/quartile range). *statistical significance p
The collagen quantification of the tissue samples is presented in Fig. 6. There were significant differences among the grafts tested, with the multifilament graphs having pronounced, but largely disorganized, collagen bundles recorded on the SEM, indicating over-scarring (Fig. 7).
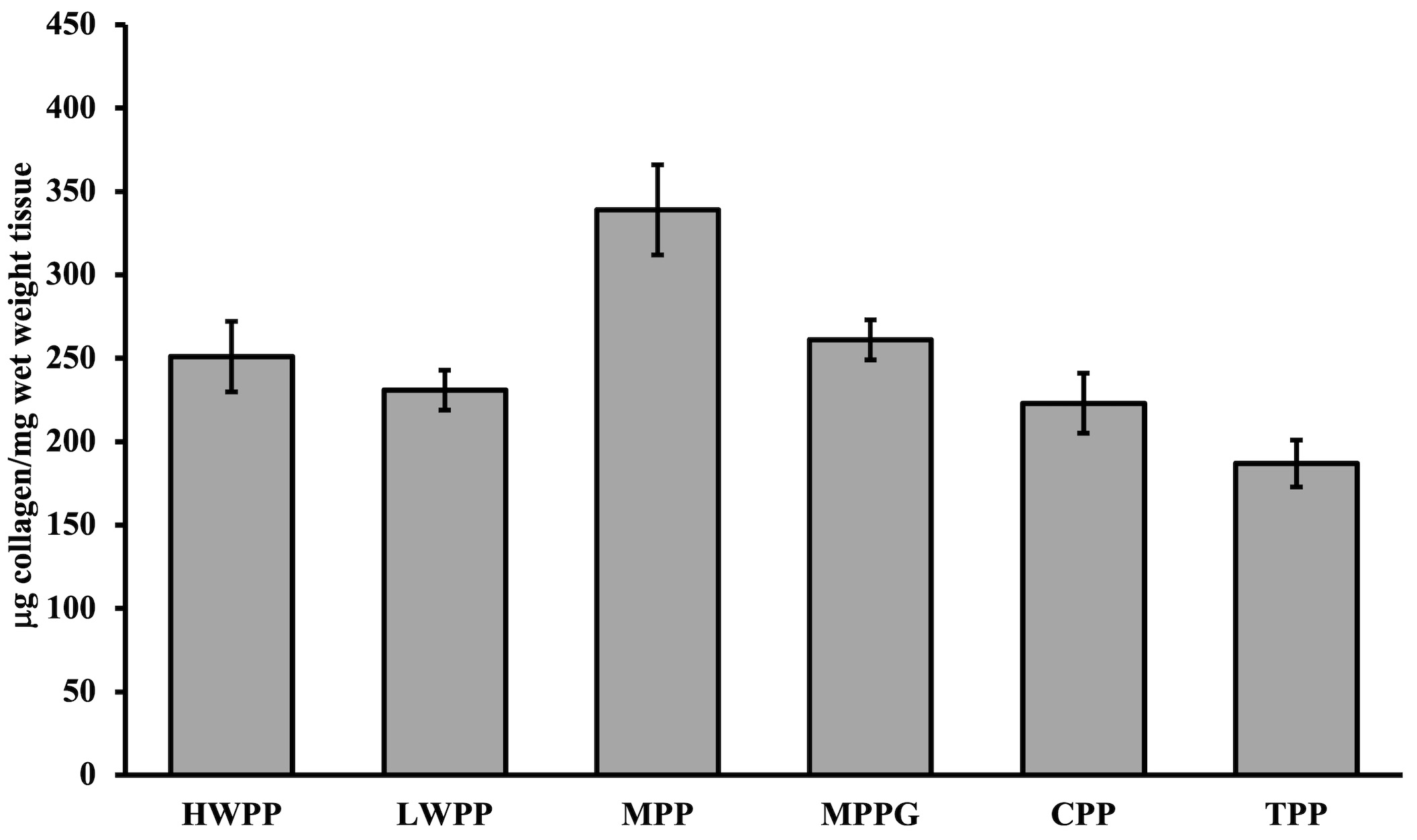 Fig. 6.
Fig. 6.Collagen quantification of explanted samples after six weeks (mean/standard error).
 Fig. 7.
Fig. 7.
Scarcely organized collagen fibers of multifilament graft
indicating over scaring (left
The amount of collagen detected varied the most between the MPP (standard error
(SE) = 27
A multivariate analysis of graft pore size, graft strength, number of inflammatory cells, collagen amount, and oxidative stress relative to abdominal wall strength after six weeks is presented in Table 2.
| Graft type | Inflammatory cells | Collagen deposited | Graft strength | Oxidative stress | Pore size |
| HWPP | 0.9971 | 0.6012 | 0.4837 | 0.0486* | 0.1019 |
| LWPP | 0.6012 | 0.5915 | 0.5951 | 0.0403* | 0.6114 |
| MPP | 0.4837 | 0.0780 | 0.9745 | 0.0471* | 0.4467 |
| MPPG | 0.6486 | 0.7403 | 0.5571 | 0.0072* | 0.4044 |
| CPP | 0.3858 | 0.7121 | 0.6372 | 0.0229* | 0.7921 |
| TPP | 0.1019 | 0.6140 | 0.4467 | 0.0044* | 0.8962 |
| *statistically significant p | |||||
The abdominal wall reinforcement was not influenced by the number of
inflammatory cells in the groups. Graft-specific variables, such as filament
thickness and pore size, also failed to present as statistically significant. The
oxidative stress induced by the grafts proved to be significant in all groups
(p
To the best of our knowledge, direct oxidative stress analysis of tissue stripped from explanted grafts has not been done before. A specific analysis of the oxidative stress levels of the tissue in direct contact with the graft indicates somewhat different results from oxidative stress as measured in blood [5]. Oxidative stress is a well-established measure and is widely used when assessing tissue cellular damage [6]. Since Spiteller reviewed the involvement of lipid peroxidation in various chronic diseases, lipid oxidation end-products have emerged as oxidative stress markers, with Trans-4hydroxy-2nonenal (4-HNE) and MDA among those most investigated [7].
The main reason for testing different grafts was to gain insight into the cellular and sub-cellular differences influenced by graft specificity. An obvious result was the positive correlation between graft weight and oxidative stress level. This result has not been presented until now, and it suggests a restrictive use of polypropylene. Bearing in mind that oxidative stress correlates positively with graft weight, one can assume that the graft weight and graft surface (as the quantity-weight of the implanted graft is increased) will determine the cellular damage induced by the oxidative stress. The mass of the graft itself is the most important independent factor affecting direct oxidative stress as expressed in the tissue in the immediate proximity. The complication rates with high-weight polypropylenes are significant, at least in vaginal surgery [8, 9, 10]. An important aspect is that the collagen and titanium coatings, intended to reduce the foreign body reaction, did little to reduce it in our study, as has also been demonstrated in other studies [11, 12]. An alternative method for decreasing the local oxidative stress could be a platelet-rich plasma covering of the polypropylene mesh, as suggested in a study by Belebecha et al. [13]. A comparison of grafts of similar weight, where one was collagen-coated,did not indicate any mitigation of the cellular oxidative stress of tissue in direct contact with the graft. A semi-absorbable multifilament mesh induced similar oxidative stress levels relative to its weight, despite the evident absorption of some filaments. Junge et al. [14] concluded that the absorbable filaments did not influence the biocompatibility, favoring our study results. Some studies have suggested that oxidative stress could be an initial factor in malignant transformation, quite apart from the inflammatory processes [15, 16, 17].
Several studies have shown that the mesh construction may be the ultimate factor in tissue ingrowth, final graft stabilization, and tissue reinforcement [18, 19, 20]. A revealing study [21], analyzing English and German literature, emphasizes graft structure and construction. Our results suggest that graft weight should be reduced to the limits of sound tissue reinforcement, while being light enough to reduce the inflammatory foreign body reaction to a minimum. The intense inflammatory response recorded with the heavier grafts in our study resulted in greater collagen deposition. These collagen deposits presented as disorganized on the electronic scan microscopy, suggesting over-scarring (Fig. 7). Collagen and collagen organization are essential for abdominal reinforcement, as demonstrated in other studies [22]. Our recent study showed that all grafts provided similar tissue reinforcement, regardless of the graft strength shown in a controlled environment [23], favoring having just the right amount of reinforcement (i.e., reducing the amount of mesh support), as mentioned above. The current study results show least oxidative stress expression with the lightest meshes, regardless of mesh construction or coating. In our opinion, the oxidative stress results indicate the need for a critically determined amount of mesh, or mesh surface, relative to the minimum needed for the reconstruction. This would permit oxidative stress and cell damage to be reduced to a minimum, allowing ingrowth and stabilization without complications. This is in contrast to other studies that have highlighted the graft structure and construction [24, 25]. In our research, graft structure did not influence the final abdominal wall reinforcement after six weeks. Aspects of graft damage by oxidative degradation highlight the oxidative stress aspect even more when a detailed chemical analysis is performed. Imel et al. [26] demonstrated damage to in vivo polypropylene by oxidative degradation by performing detailed chemical analysis as well as by using the electron microscope. However, the statements “polypropylene is highly susceptible to the oxidative effects of the metabolites produced by phagocytic cells during inflammatory response” and “These byproducts of the inflammatory response may degrade and embrittle the material causing it to become rigid” are open to question. Mesh degradation, reported to cause surface cracking, mesh contraction, loss of mass, decreased melting temperature, embrittlement, and reduced compliance of the polypropylene, is directly influenced by oxidative stress that is induced locally through chemical degradation [25]. In vivo degradation of both hernia and pelvic meshes has been demonstrated in several studies that question the inertness of implanted polypropylene [27, 28, 29, 30].
A recent study by Poppas et al. [31] reports that a hydrogel coating reduces oxidative stress significantly, but this type of mesh coating is not available in our country. They measured 8-hydroxydeoxyguanosine (8-OHDG), an intracellular oxidative stress marker known to adhere to DNA lesions created by oxidative stress. The lower oxidative stress levels they report differ significantly from our results, but it is necessary to bear in mind the different coating and the oxidative stress markers that were measured.
This is an experimental animal study and the results might be different in the human. The follow-up period of six weeks might be considered too short, leaving uncertainty as to whether the results would remain the same after a longer follow-up period. The experimental animal study used abdominal wall defects as a model for testing grafts used in vaginal surgery.
In our experimental animal study, tissue-induced oxidative stress levels were negative predictors for urogynecology synthetic graft tissue reinforcement. The mechanical strength of the graft was not relevant either to the process of stabilization or to the quality of the final tissue reinforcement. According to our experimental animal study results, the expression of oxidative stress presented with a positive correlation to graft weight.
PM—idea, writing of the manuscript, correcting, statistical analysis; II—structure corrections, language editing, statistical corrections; VB—provided help and advice on the analysis. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
All experimental procedures that involved animals were conducted in compliance with the European Council Directive (EU directive of 2021; 2010/63/EU) and Guide for the Care and Use of Laboratory animals (10th edition, National Academy Press) and ARRIVE guidelines 2.0. The Ethics Committee for Animal Experimentation of the Faculty of Medicine, University of Nis approved (No 2477-19) the experimental study.
The authors would like to thank the Ministry of Education, Science and Technological Development of Republic of Serbia (Grant No: 451-03-9/2021-14/200113) for financial support.
The research was funded by the Ministry of Education, Science and Technological Development of Republic of Serbia (Grant No: 451-03-9/2021-14/200113).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
