Academic Editor: Michael H. Dahan
Background: Previous studies shown that RNA binding motif proteins
(RBM) participate in regulating various physiological processes such as cell
autophagy, proliferation, and apoptosis, and are abnormally highly expressed in
placental trophoblast cells intervened by hypoxia in vitro, but their molecular
mechanisms regulating placental trophoblast damage remain unclear. This study
aims to investigate the role and molecular mechanism of RBM10 in regulating
hypoxia-induced placental trophoblast injury through endoplasmic reticulum
stress. Methods: CCK-8 cell proliferation assay and Transwell cell
invasion assay were applied to detect the proliferation and invasion ability of
normal, hypoxic and RBM10 up-regulated plus hypoxic embryonic trophoblast cells,
respectively. The expression of endoplasmic reticulum stress-related proteins
(ERN-1) and C/EBP homologous protein (CHOP), apoptosis-related proteins B cell
lymphoma-2 (Bcl-2), Bcl-2-associated X protein (Bax) and Caspase-3, and
autophagy-related proteins including microtubule-associated protein 1 light chain
3 (LC-3), Beclin-1 and P62 were also detected by western blot assays. The effects
of hypoxia and overexpression of RBM10 on placental trophoblast apoptosis were
examined using flow cytometry. Results: We found that the growth and
invasion ability of placental trophoblast cells treated with hypoxia were
significantly decreased (p
The process of fertilized egg implantation and embryo formation includes embryonic adhesion to the uterine wall, proliferation and migration of embryonic trophoblast cells, and invasion of trophoblast cells into the endometrium [1, 2], abnormalities in this process can cause placental trophoblast hypoxia, leading to placental ischemic disease, which can lead to placental ischemic disease (PID), such as intrauterine growth retardation (IUGR), placental abruption, and preeclampsia (PE) [3, 4]. PE is an idiosyncratic condition during pregnancy and is an important cause of maternal and fetal morbidity and mortality [5, 6], and delivery of the placenta and fetus is the only way to cure PE [7]. It is estimated that PE occurs in 3%~7% of pregnant women worldwide, and the exact pathogenesis of preeclampsia remains unclear to date [8].
Compared to normal pregnancy, there is significant apoptosis of placental trophoblast cells in preeclampsia, and the expression of genes with anti-apoptotic effects is significantly downregulated [9]. The placenta as an important organ for maintaining pregnancy undergoes a huge growth change process throughout pregnancy, and the balance of cell proliferation and apoptosis is the basis to ensure the normal role of the placenta, and dysregulation of apoptosis will lead to pathological pregnancy. It has been shown that placental endoplasmic reticulum stress (ERS) is closely related to the pathophysiological mechanisms of PE and is an important intermediate in the pathogenesis of PE [10]. There are many molecular chaperone proteins in the endoplasmic reticulum that play an important role in synthesizing and packaging proteins during the endoplasmic reticulum stress state. Under normal conditions, endoplasmic reticulum stress protects the organism from injury, but increased or prolonged endoplasmic reticulum stress results in the activation of specific apoptosis-related signaling pathways [11], accompanied by programmed cell death, and ERS is thought to be a novel pathway for regulating apoptosis [12].
RNA motif binding proteins (RBM) are a family of proteins with a stable three-dimensional structure that recognize and bind to RNA, and are involved in the regulation of various physiological or pathological processes such as cell autophagy, proliferation, and apoptosis [13, 14]. Numerous studies have found striking similarities in the biological properties of normal placental trophoblasts and tumor cells [15]. Some studies have reported that RBM10 and related proteins are differentially expressed in glioma cells as well as lung adenocarcinoma cells and have an important regulatory role in apoptosis [16, 17]. Other groups have also found that RBM-related proteins are abnormally highly expressed in placental trophoblast cells intervened by hypoxia in vitro and induce autophagy and apoptosis, promoting the development of PE, but the specific role in PE development and progression has not been further explored [16, 18], and whether it regulates trophoblast apoptosis through activation of the endoplasmic reticulum stress pathway needs to be further investigated.
In this study, we observed the changes of endoplasmic reticulum stress, apoptosis and autophagy in trophoblast cells induced by hypoxia using the trophoblast cell line HTR-8 as the experimental object, and further analyzed the changes of endoplasmic reticulum stress, apoptosis and autophagy in trophoblast cells after the upregulation of RBM10 expression, aiming to explore the role and mechanism of RBM10 in regulating cell injury and provide a new basis for exploring the pathogenesis of PE and its early prediction and diagnosis.
HTR-8 cells were obtained from the Institute of Cell Research, Chinese Academy
of Sciences (Shanghai, China). Cells were cultured in RPMI-1640 medium containing
10% FBS at 37 °C in 5% CO
Total proteins were extracted from the above three groups of embryonic trophoblast cells using RIPA buffer and protease inhibitor (CW2200S, CWBIO, Taizhou, Jiangsu, China), respectively, the tissues were lysed, and the cells were centrifuged (12 000 r/min) at 4 °C for 20 min, separated by 10% gel electrophoresis, and then transfected onto PVDF membranes (IPVH00010, Millipore, Burlington, MA, USA). The PVDF membranes were transferred in an ice bath at 350 mA with a constant flow, and the RBM10 protein was transferred for 3 h. Afterwards, the PVDF membranes were removed and placed in a closure solution (5% skim milk) and closed in a shaker at room temperature for 1 h. Then, the membranes were incubated with the corresponding primary antibody (1:1000) for 12 h at 4 °C and then with the secondary antibody (1:1000) for 1 h at room temperature. All Western blots were repeated 3 times using Tublin as an internal reference. Changes in protein levels were expressed by protein bands in grayscale values, and the data were processed by Image J software (version 1.8.0, LOCI, University of Wisconsin, Madison, WI, USA) and represented as bar graphs. Antibodies were as follows: rabbit anti-C/EBP-homologous protein (CHOP) (SAB4500631), rabbit anti-endoplasmic reticulum to nucleus signaling 1 (ERN-1) (HPA027730), mouse anti-microtubule-associated protein 1 light chain 3 (LC-3) (WH0081631M1), rabbit anti-Beclin-1 (PRS3611), rabbit anti-P62 (P0067), rabbit anti-Bcl-2 (B cell lymphoma-2) (SAB4500003), rabbit anti-Bcl2-associated X protein (Bax) (SAB5701392) and rabbit anti-Active-Caspase3 (MAB10753) (Sigma, St. Louis, MO, USA).
Transwell chambers were placed in 24-well plates, and 50
The above three groups of embryonic trophoblast cells at logarithmic growth
stage were digested by trypsin and made into single cell suspensions, which were
counted and inoculated in 96-well plates with 100
Apoptosis was detected with Annexin V-FITC Apoptosis Detection Kit I (331200, Thermo Fisher Scientific, Richardson, TX, USA). Annexin V binding buffer was added to the recovered cells. Annexin V/FITC mix was then added to the cell suspension. PI dye was incubated in the dark for 15 min before apoptosis analysis was performed by flow cytometry. This experiment was performed three times.
All experimental data in this study were performed using SPSS 18.0 software (IBM
Corp., Chicago, IL, USA). The one-way ANOVA analysis was done to determine any
difference between two groups, and differences between groups further assessed
using Student T-test. Data are expressed as
In this experiment, the effect of RBM10 on cell proliferation was measured by
CCK-8 assay. The subjects were divided into three groups: normal embryonic
trophoblast (NC), hypoxic placental trophoblast (Hypoxia) and hypoxic embryonic
trophoblast with upregulated RBM10 expression (RBM10-OE). The OD values of the
three groups of cells on the first day were 0.432
This result indicates that the cell growth and viability of hypoxia-treated embryonic trophoblast cells was reduced compared with the control cells, and the cell growth and viability of hypoxic embryonic trophoblast cells overexpressing RBM10 was further reduced (Fig. 1). This indicates that both RBM10 overexpression and hypoxia have an inhibitory effect on the cell growth and viability.
 Fig. 1.
Fig. 1.Effect of RBM10 on the proliferation ability of embryonic trophoblast cells.
The results of Transwell experiments showed that the number of cells invading to
the lower chamber surface of Transwell was significantly reduced in the
hypoxia-treated cells compared to the NC group of trophoblast cells, and the
invasion ability of hypoxic embryonic trophoblast cells with up-regulated RBM10
expression was further reduced. The differences were statistically significant
(p
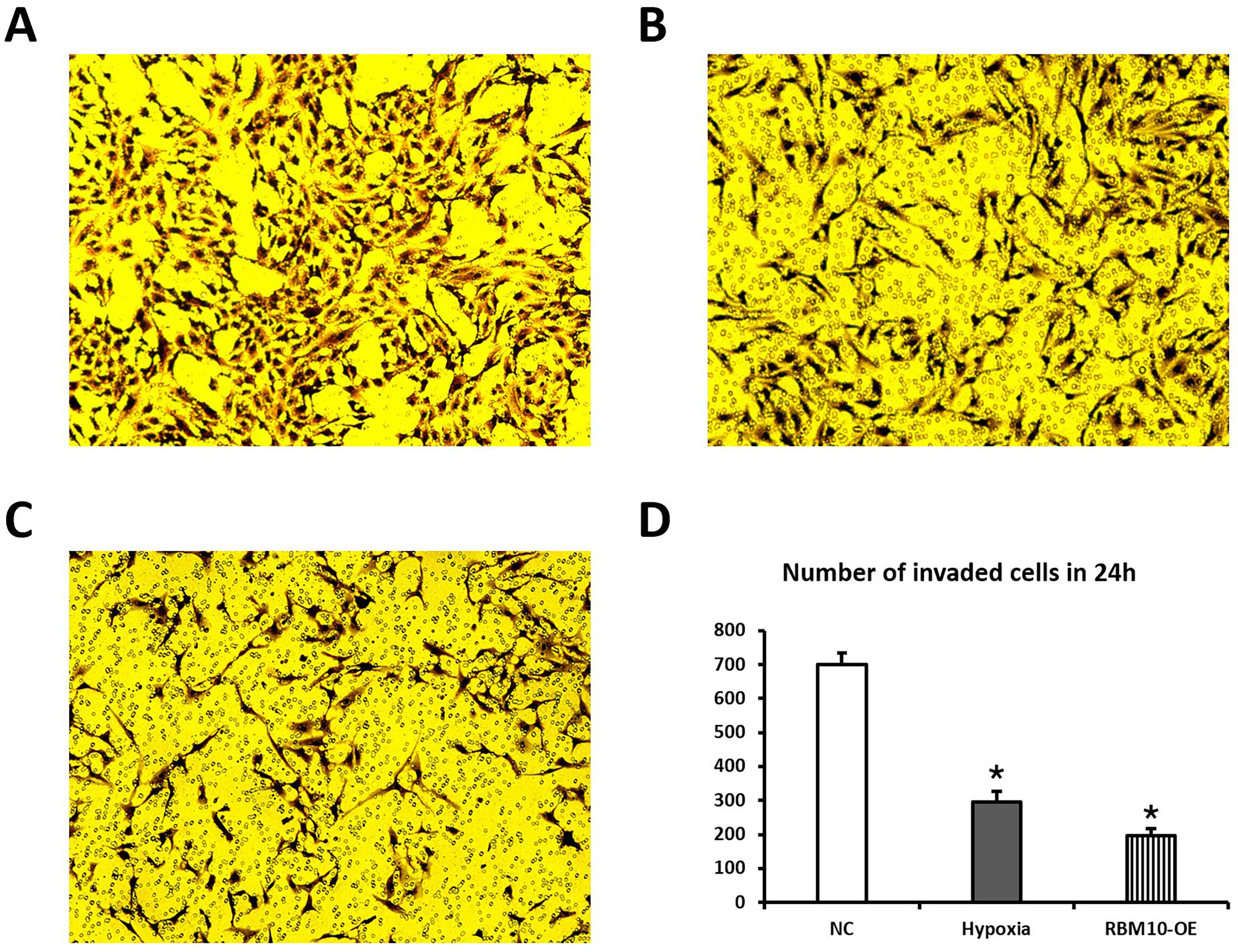 Fig. 2.
Fig. 2.Effect of transfection with RBM10 on cell invasion ability. (A–C) Transwell assay with three sets of data for normal embryonic trophoblast (NC), hypoxic placental trophoblast (Hypoxia) and hypoxic embryonic trophoblast with upregulated RBM10 expression (RBM10-OE). (D) Statistical comparison of the invasion ability of the three groups of cells.
To further investigate the mechanism of RBM10-induced apoptosis in HTR-8 cells,
the expression levels of ERN-1 and CHOP, markers of endoplasmic reticulum stress,
were examined in this study. western blot results showed that the expression
levels of ERN-1 and CHOP were differentially upregulated in the experimental
group compared with the control group (p
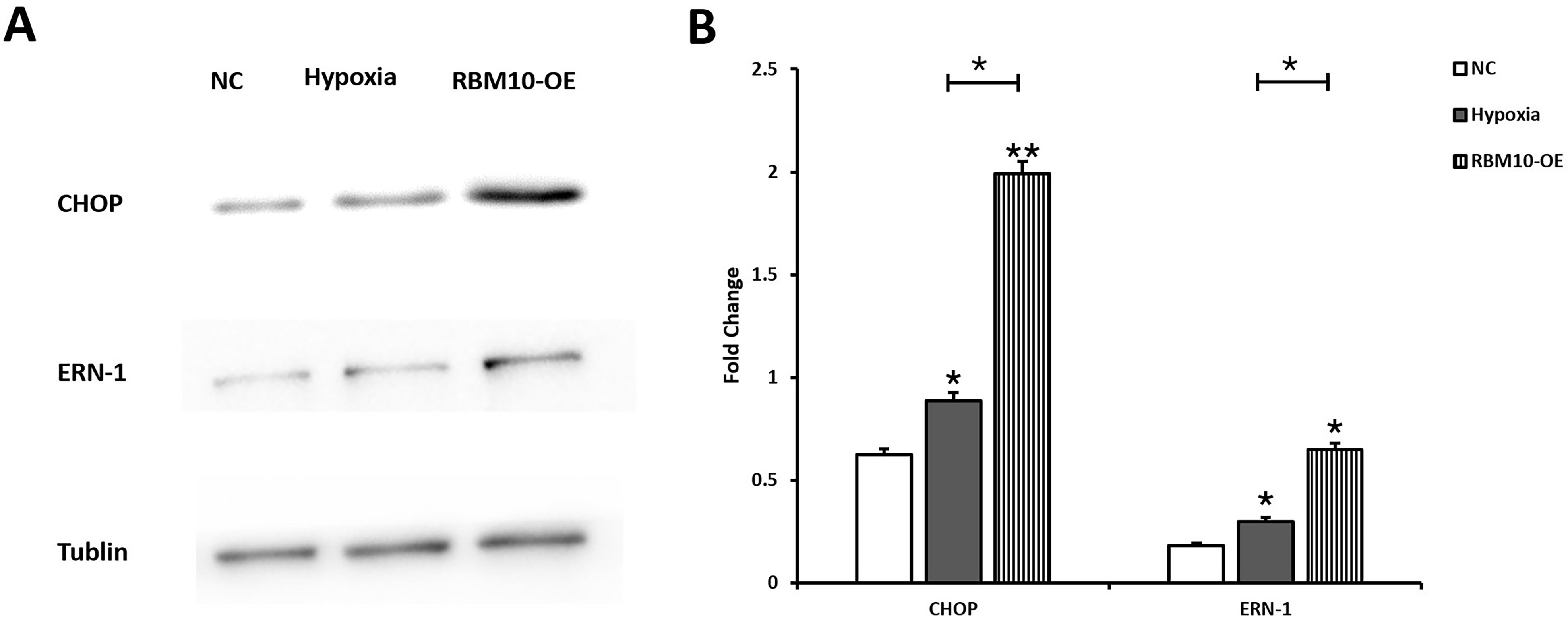 Fig. 3.
Fig. 3.Effect of RBM10 on the expression levels of endoplasmic
reticulum stress-related proteins in HTR-8 cells. (A) Expression levels of CHOP
and ERN-1 in the NC, Hypoxia and RBM10-OE groups were detected by western blot
assay. (B) Image J software was used to analyze the signals, the relative protein
expression was normalized by Tublin expression, * p
Annexin V-FITC apoptosis assay suggested that the apoptosis rate of
hypoxia-stimulated trophoblast cells was significantly increased, and the
difference was statistically significant (p
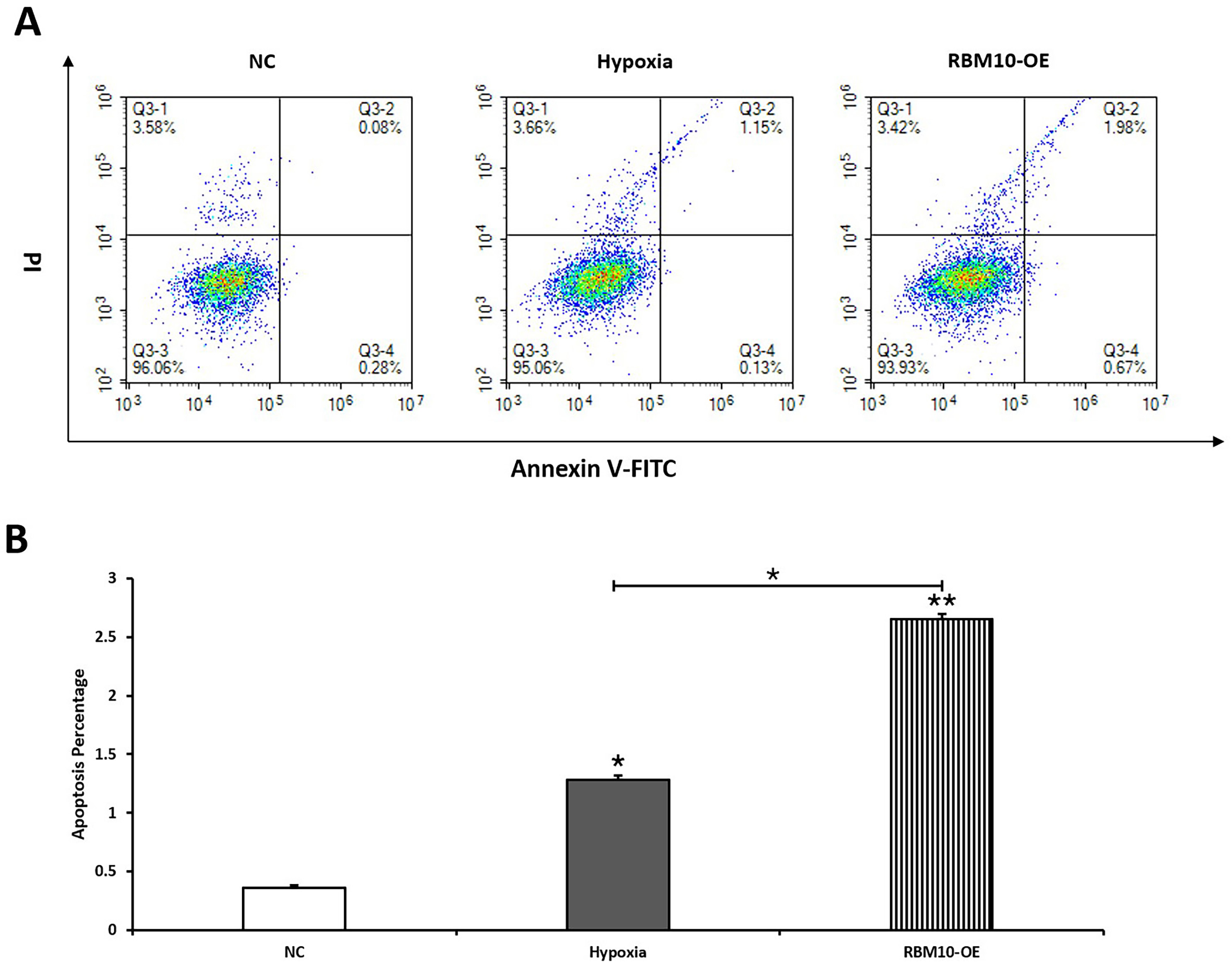 Fig. 4.
Fig. 4.The effect of RBM10 on the apoptotic function of trophoblast
cells detected by flow cytometry. (A) Flow cytometry assay detected the
percentage of apoptotic cells in the NC, Hypoxia, and RBM10-OE groups. (B) The
early and late apoptosis were quantified, * p
The effect of RBM10 on the expression levels of placental trophoblast
apoptosis-related proteins Caspase-3, Bcl-2 and Bax was also further
investigated. The results showed that the expression of Bcl-2 was 1.532
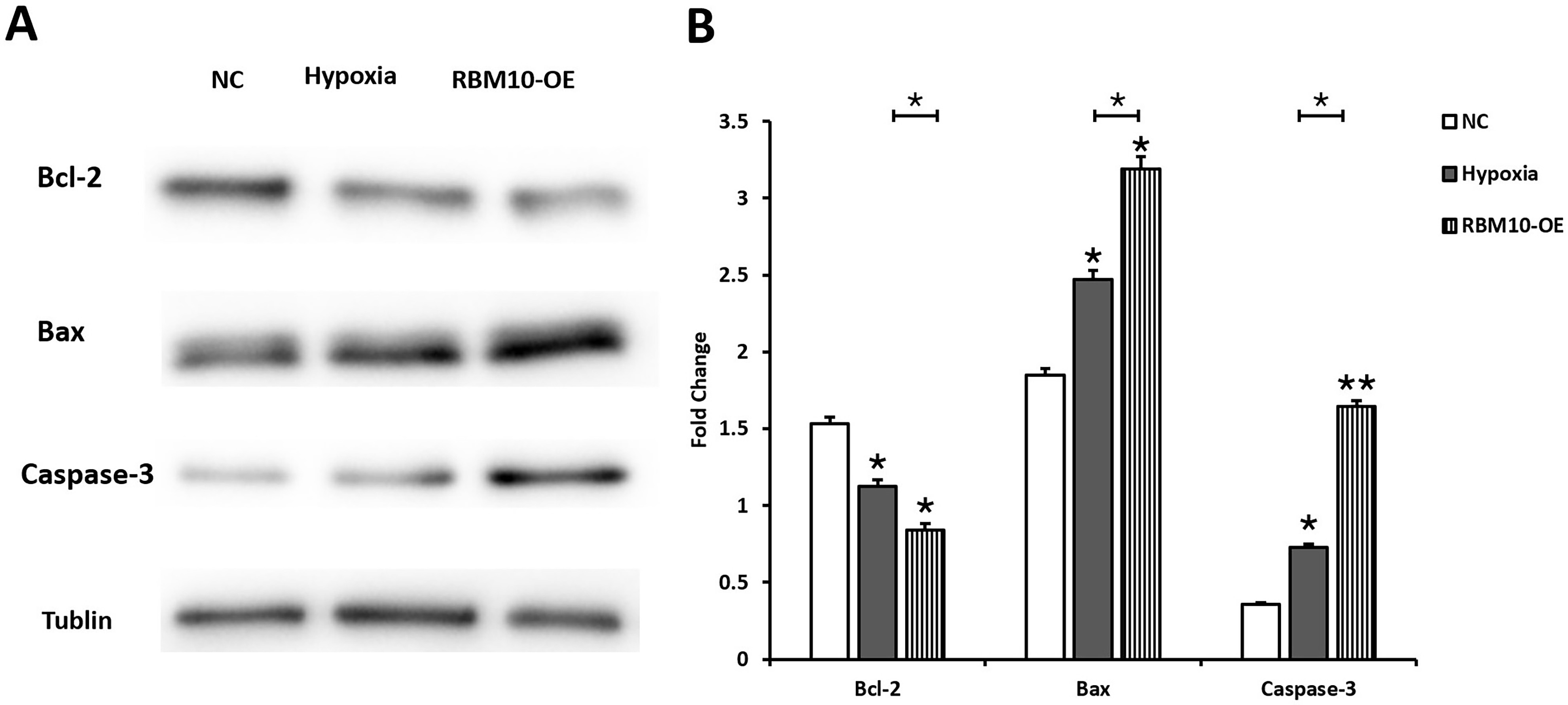 Fig. 5.
Fig. 5.Effect of RBM10 on the expression level of apoptosis-related
proteins in HTR-8 cells. (A) Western blot assay detected the expression of Bcl-2,
Bax, as well as Caspase-3 in the NC, Hypoxia, and RBM10-OE groups. (B) Image J
software was used to analyze the signals, the relative protein expression was
normalized by Tublin expression, * p
Further, we explored the effect of RBM10 on the expression levels of placental
trophoblast autophagy-related proteins LC-3, Beclin-1 and P62. The results showed
that compared with the normal group, the expression levels of autophagy marker
proteins LC-3 and Beclin-1 were significantly increased in HTR-8 after 48 h of
hypoxia treatment, while the expression levels of P62 were significantly
decreased, and the differences were all statistically significant (p
 Fig. 6.
Fig. 6.Effect of RBM10 on the expression levels of autophagy-related
proteins in HTR-8 cells. (A) Western blot assay detected the expression of LC-3,
Beclin and P62 in the NC, Hypoxia, and RBM10-OE groups. (B) Image J software was
used to analyze the signals, the relative protein expression was normalized by
Tublin expression, * p
PE is a serious pregnancy complication with an incidence of approximately 2%~8% of all pregnancies and may result in maternal and fetal death [19]. Currently, the etiology and pathogenesis of PE are unclear, and iatrogenic termination is the only treatment for PE [20]. Existing studies have shown that in the early stages of pregnancy, embryonic development and placental formation take place under relatively hypoxic conditions, and trophoblast blockage of the uterine spiral artery causes a hypoxic environment that can promote vascular recasting and trophoblast differentiation and invasion, thus ensuring normal pregnancy; however, when hypoxia is excessive, it causes chronic ischemia and hypoxia in the placenta in late pregnancy, leading to PE, fetal growth restriction, miscarriage, stillbirth, and other adverse pregnancy outcome [21, 22, 23]. During early pregnancy, trophoblast cells infiltrate into the maternal uterus to form the placenta through tumor-like properties (high proliferation and high infiltration). However, the mechanisms by which hypoxia regulates the physiological function of placental trophoblast cells are not known [24], and placental trophoblast apoptosis may be a key factor in the pathogenesis of PE.
RNA motif binding proteins recognize and bind to RNA and play an important role in RNA translation, shearing and maintaining RNA stability. In addition, it was found that RBM-related proteins are also closely associated with tumor development and prognosis, and RBM10 is associated with the expression of apoptotic genes such as caspase-3 and Bax, which are key regulators of apoptosis [17]. In this study, we used a cellular hypoxic chamber device to simulate a PE hypoxic environment and constructed an RBM10 overexpression system to observe the effects of overexpression of RBM10 and hypoxia on the proliferation and invasion capacity, endoplasmic reticulum stress, apoptosis and autophagy of HTR8 cells. We found that hypoxia inhibited the proliferation and invasion ability of HTR-8 cells, and led to a significant increase in apoptosis. In addition, we found that overexpression of RBM10 further inhibited the growth and migration of HTR-8 cells and further promoted hypoxia-induced apoptosis in HTR-8 cells.
Endoplasmic reticulum stress is a prevalent stress-defense mechanism in eukaryotic cells [25], and in the ERS state, cells initiate the Unfolded Protein Response (UPR) to enhance the folding of unfolded proteins and the degradation of misfolded proteins to restore the normal physiological function of the endoplasmic reticulum [26]. Some stimuli that trigger ERS also induce cellular autophagy. Autophagy, as a conserved degradation mechanism in eukaryotic cells, can reduce ERS levels by accelerating the degradation of misfolded proteins and is another important role in helping the endoplasmic reticulum to restore homeostasis following UPR [27]. However, prolonged UPR activation, which may trigger terminal UPR, leads to apoptosis [28, 29]. And it is still unclear whether pre-eclamptic trophoblast apoptosis is caused by inducing endoplasmic reticulum stress. changes in the expression of CHOP and ERN-1, which are key regulatory molecules of endoplasmic reticulum stress, are often used as markers of endoplasmic reticulum stress.
It has been shown that hypoxia causes the accumulation of non-normally folded
proteins in the endoplasmic reticulum, which inhibits the synthesis of normal
proteins and eventually triggers endoplasmic reticulum stress. When ERS occurs,
the dynamic balance of Ca
In conclusion, the results of this study show that overexpression of RBM10 induces apoptosis in trophoblast cells and reduces the proliferation capacity of trophoblast cells, and its mechanism of apoptosis induction may be related to the endoplasmic reticulum stress pathway. The findings provide a new perspective for understanding the etiology and pathogenesis of PE, and provide a new breakthrough for the early prediction and diagnosis of PE.
XC—extraction and drafting of the manuscript; XC, JD, LYC, LLW and GX—analysis of data, manuscript revision; GX—design and revision, statistical analysis. All authors read and approved the final manuscript.
The Ethics approval has been exempted by the Research ethics committee of the Second Hospital of Shandong university.
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
