†These authors contributed equally.
Academic Editor: Gianluca Caruso
Background: Over the
past several decades, rates of cesarean delivery have increased considerably
worldwide. As cesarean section (CS) may also result in changes
to uterine position, the relationship between delivery modes, postpartum uterine
position and cesarean scar defect (CSD) warrants elucidation. Materials
& Methods: Here, we conducted a retrospective observational study
evaluating 921 women (482 underwent vaginal delivery and 439 underwent CS) who
had undergone transvaginal ultrasonography (TVU) early in their pregnancy (
The uterus is a dynamic, pear-sharped organ and plays a variety of functions in pregnancy and delivery. Supported by surrounding ligaments, its natural position can be classified as anteverted or retroverted [1]. Uterine anteversion is not equivalent to anteflexion, but define uterus anteflexed or retroflexed by ultrasound is more simply and practical.
Under normal conditions, the uterus is generally anteflexed. Uterine retroflexion is a less frequent normal presentation of the uterus. seen in approximately 20% of women (range 10–38%), which is reported to be associated with dyspareunia (66.7% vs. 42.1%), dysmenorrhea (66.7% vs. 42.9%) and uterine prolapse (Odds Ratio (OR) = 4.5) as compared with anteflexion [2, 3, 4].
Throughout the processes of pregnancy and vaginal delivery, uterine position changes significantly. The postpartum uterus will be mostly retroverted on postpartum days 1–3, slowly progressing to anteversion from days 7–14 until day 56 [5].
Over the recent decades, the application of cesarean section (CS) has considerably increased worldwide [6, 7]. Consequently, the incidence of long-term CS complications, such as that of cesarean scar defects (CSD), has also increased. After vaginal delivery, CS may result in changes in uterine position. An anteverted, retroflexed uterus is a common consequence of CS and manifests with changes in the uterine angle and a more retroflexed position than after vaginal delivery [8, 9]. Some studies have suggested that retroflexed uterus is more likely to manifest with CSD; van der Voet LF et al. [10] attributed this phenomenon to the impairment of wound healing and consequent counteracting forces about the uterine scar [10, 11, 12, 13]. To date, the natural history of CSD formation remains unclear. Here, we conducted a retrospective observational study to compare the changes in uterine flexion among women who underwent CS and vaginal delivery and investigated CSD occurrence.
We assumed that both delivery modes result in uterine retroflexion but that CS predisposes it to a greater degree. We attempted to determine whether anteversion or retroversion more frequently associates with CSD.
Participants were recruited from January 2018 to December 2018 at the Department of Obstetrics, International Peace Maternity and Child Health Hospital (IPMCH) in Shanghai, China.
Sample size calculation was performed according to our pilot study about the prevalence of CSD, which showed the incidence of CSD were 60% and 40% in anteflexion and retroflexion, respectively. A power calculation with PASS sample prediction software version 11.0 (NCSS, Kaysville, UT, USA) showed that 340 post-CS women (alpha error = 0.05, beta = 0.20) were required. Therefore 340 women with vaginal delivery were also needed (ratio 1:1).
Women with a singleton delivery (greater than 36 weeks gestational age) without previous CS and whose uterine position had been confirmed early in pregnancy (at about 8 weeks) by transvaginal ultrasonography (TVU) were included.
Exclusion criteria included: (1) Patients with previous CS or other uterine surgeries. (2) Patients with uterine anomalies (such as uterine malformation, adenomyosis, or myoma of uterus), placenta previa and CS incisional lacerations. (3) Patients with severe gestational complications (e.g., HELLP syndrome, non-reproductive tract infections). (4) Patients with systematic disease requiring complex treatment or conditions that significantly affect wound healing (e.g., systemic lupus erythematosus). (5) Patients with an inability to provide consent or adhere to study protocol.
Uterine flexion was defined as the angle between the cervical longitudinal axis and the uterine longitudinal axis in the sagittal plane [14]. Uterine anteflexion was defined as anterior deviation of the uterine longitudinal axis in relation to the cervical axis while the uterine was posteriorly retroflexed [4]. CSD measurement was performed according to previously described guidelines [15].
Early pregnancy TVU images were extracted from the hospital picture archiving and communication system (PACS) and reviewed by another sonographer. All TVU images were obtained either using the same device or equipment producing comparable imaging quality. Imaging was performed by a sonographer who was blinded to clinical information and prior TVU data.
Women who underwent CS were routinely operated on via a lower uterine section incision that was followed by a double-layer uterine closure with the decidua included in the suturing of the first layer.
The primary outcome is the uterine position for all of the women. All women underwent additional TVU imaging for evaluation of uterine position around 3–6 months postpartum. The secondary outcome is the CSD occurrence for women underwent CS. Screening for CSD was performed by SIS in post-CS women after the standard TVU. Imaging was performed according to guidelines reported previously by Naji et al. [10, 14].
Data concerning body mass index (BMI) and gestational complications (e.g., gestational diabetes mellitus (GDM), hypertension, anemia) were recorded. Perioperative conditions including cervical dilation, premature rupture of membranes (PROM), intrauterine infection and emergency CS were also recorded.
Differences in epidemiological factors (e.g., age, gestational age) between
vaginal and CS delivery groups were compared using an independent sample
T-test. Changes in uterine flexion from early pregnancy to the
postpartum in both groups were compared using the
Chi-squared test, as were group differences in
early pregnancy and the postpartum. The relationship between the incidence of CSD
and postpartum uterine position was also analyzed using the Chi-squared test. The
variables distributed normally were presented as median
A total of 921 women with singleton pregnancies were initially recruited for this study; 716 women (371 of whom underwent vaginal and 345 of whom underwent CS delivery) (Fig. 1).
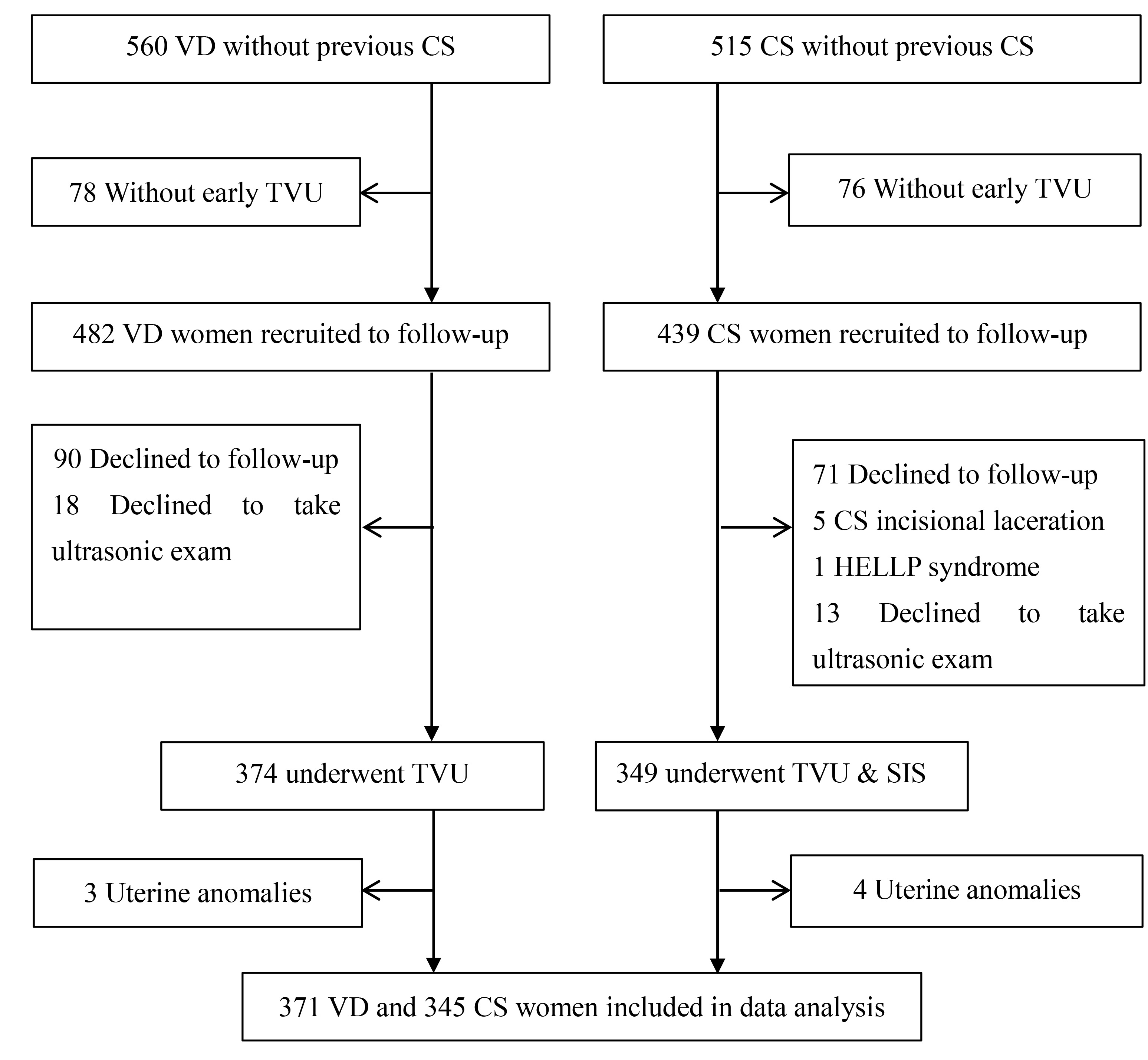 Fig. 1.
Fig. 1.Study flowchart. VD, vaginal deliveries; CS, cesarean section; HELLP syndrome, Hemolysis Elevated Liver Enzymes Low Platelets syndrome; TVU, transvaginal ultrasonography; SIS, saline infusion sonohysterography.
Patients’ age was significantly higher (30.18
| Vaginal Delivery (n = 371) | Cesarean Section (n = 345) | p value | ||
| Age (years) | 30.18 |
31.70 |
||
| Gestational age (weeks) | 39.13 |
38.71 |
0.013 | |
| Parity (times) | 0.457 | |||
| 0 | 311 (83.8) | 293 (84.9) | ||
| 1 | 56 (15.1) | 51 (14.8) | ||
| 2 | 4 (1.1) | 1 (0.3) | ||
| Total preterm (times) | 0.300 | |||
| 0 | 371 (100.0) | 344 (99.7) | ||
| 1 | 0 (0.0) | 1 (0.3) | ||
| Gravidity (times) | 0.364 | |||
| 0 | 218 (58.8) | 225 (65.2) | ||
| 1 | 108 (29.1) | 75 (21.7) | ||
| 2 | 22 (5.9) | 22 (6.4) | ||
| 3 | 14 (3.8) | 16 (4.6) | ||
| 4 | 4 (1.1) | 5 (1.4) | ||
| 5 | 2 (0.5) | 1 (0.3) | ||
| 6 | 3 (0.8) | 1 (0.3) | ||
| Early pregnancy uterine position | 0.990 | |||
| Anteflexion | 304 (81.9) | 283 (82.0) | ||
| Retroflexion | 67 (18.1) | 62 (18.0) | ||
| Data are mean p value represents the significance between the vaginal delivery group and cesarean section delivery group. Numeric data compared for mean equality via independent samples Student’s t tests (age and gestational age) and the categorical data compared for distributional equality via Pearson’s chi-square or Fishers Exact tests. *Statistically Significant p | ||||
Uterine flexion was similar among both CS and vaginal delivery groups in early pregnancy; anteflexion and retroflexion rates were 81.9% and 18.1% in vaginal, and 82.0% and 18.0% in CS delivery groups, respectively. Anteflexion and retroflexion rates were 70.9% and 29.1% in vaginal, and 53.3% and 46.7% in CS delivery groups in the postpartum, respectively (Table 2).
| Postpartum | p | |||
| Anteflexion | Retroflexion | |||
| Early pregnancy | Vaginal delivery | |||
| Anteflexion | 256 | 48 | ||
| Retroflexion | 7 | 60 | ||
| Total | 263 (70.9) | 108 (29.1) | ||
| CS | ||||
| Anteflexion | 173 | 110 | ||
| Retroflexion | 11 | 51 | ||
| Total | 184 (53.3) | 161 (46.7) | ||
| Data are n, n (%).
p represents the difference in postpartum uterine position between vaginal delivery and CS. Categorical data compared for distributional equality via Pearson’s chi-square. | ||||
Uterine positions significantly changed to retroflexed in both CS (p
Among the 345 women who underwent CS and postpartum SIS follow-up, 146 were found to have suffered a CSD. Prior delivery mode(s), GDM, PROM, pre-operative intrauterine infection, cervical dilation, emergency operation and postpartum anemia did not significantly affect the prevalence of CSD. The prevalence of CSD showed no significantly difference with the postpartum position of the uterus, as shown in Table 3. CSD Prevalences in anteflexion and retroflexion uterus were found to be 46.7% (95% CI 39.5%–54.0%) and 37.3% (95% CI 29.7%–44.8%), respectively (p = 0.08). Changes in uterine position, or lack thereof, did not affect CSD prevalence (p = 0.326 and p = 0.52, respectively). These data are shown in Table 4.
| NCSD (199) | CSD (146) | Prevalence (95% CI) | p value | ||
| GDM | 0.69 | ||||
| YES | 27 | 22 | 44.9 (30.5–59.3) | ||
| NO | 172 | 124 | 41.9 (36.2–47.6) | ||
| PROM | 0.14 | ||||
| YES | 47 | 25 | 34.7 (23.5–46.0) | ||
| NO | 152 | 121 | 44.3 (38.4–50.3) | ||
| Pre-operation intrauterine infection | 0.34 | ||||
| YES | 21 | 11 | 34.4 (17.0–51.8) | ||
| NO | 178 | 135 | 43.1 (37.6–48.7) | ||
| Cervical Dilation | 0.24 | ||||
| 177 | 135 | 43.3 (37.7–48.8) | |||
| 5 | 5 | 50.0 (12.3–87.7) | |||
| 17 | 6 | 26.1 (6.7–45.4) | |||
| Emergency operation | 0.20 | ||||
| YES | 101 | 64 | 38.8 (31.3–46.3) | ||
| NO | 98 | 82 | 45.6 (38.2–52.9) | ||
| Postpartum anemia | 0.91 | ||||
| NO | 120 | 89 | 42.6 (35.8–49.3) | ||
| Anemia | 79 | 7 | 41.9 (33.5–50.3) | ||
| GDM, gestational diabetes mellitus; PROM, premature rupture of membranes; NCSD,
no cesarean scar defect.
Categorical data compared for distributional equality via Pearson’s chi-square or Fishers Exact tests. | |||||
| NCSD (199) | CSD (146) | Prevalence (95% CI) | aOR (95% CI) | p value | ||
| Early pregnancy uterine position | 0.83 | |||||
| Anteflexion | 164 | 119 | 42.1 (36.3–47.8) | ref | ||
| Retroflexion | 35 | 27 | 43.6 (30.9–56.2) | 1.1 (0.6–1.9) | ||
| Postpartum uterine position | 0.08 | |||||
| Anteflexion | 98 | 86 | 46.7 (39.5–54.0) | ref | ||
| Retroflexion | 101 | 60 | 37.3 (29.7–44.8) | 0.7 (0.4–1.0) | ||
| Uterine position not changed | 0.52 | |||||
| A-A | 93 | 80 | 46.2 (38.7–53.8) | ref | ||
| P-P | 30 | 21 | 41.2 (27.2–55.2) | 0.8 (0.4–1.5) | ||
| Uterine position changed | 0.33 | |||||
| A-P | 71 | 39 | 35.3 (26.4–45.5) | ref | ||
| P-A | 5 | 6 | 54.6 (19.5–89.6) | 2.2 (0.6–7.6) | ||
| A-A represents anteflexion both in early pregnancy and the postpartum; A-P
represents anteflexion in early pregnancy and retroflexion in the postpartum; P-P
represents retroflexion both in early pregnancy and the postpartum; P-A
represents retroflexion in early pregnancy and anteflexion in the postpartum.
NCSD- no cesarean scar defect.
Categorical data compared for distributional equality via Pearson’s chi-square or Fishers Exact tests. | ||||||
In this retrospective case control study, we found that the uterus of post-CS patients was retroflexed to a more significant proportion when compared to that of patients who had undergone vaginal delivery. These findings are in line with previous studies. Anteflexed uterus was found more likely to manifest with CSD.
Mulic-Lutvica et al. [5] classified the post-vaginal delivery uterus as retroverted, upright and anteverted, noting that the uterus typically changes from retroverted to anteverted within 6–8 weeks, as studied in 42 women with uncomplicated vaginal term deliveries. Sanders et al. [8] classified uterine position into 7 types according to a combination of anteverted or retroverted, anteflexed or retroflexed, or midline in a cross-sectional study. Compared with parous and nulliparous women, patients who had undergone CS had greater rates of anteverted, retroflexed uterus. Kaelin et al. [9] reported in a retrospective cohort study classified uterine position according to flexion angles between the longitudinal axis of the uterine body and the cervix, reporting that CS alters the angle of uterine flexion (i.e., to a more retroflexed position) while vaginal delivery does not, although not significantly. Al Naimi et al. [16] previously reported that the uterus was anteflexed in 82.5% and retroflexed in 17.5% in post-cesarean women (N = 200), which was contradicts with our result, while they also did not found significant correlation between the position of uterus and the presence of CSD. As we merely defined uterine position as either anteflexed or retroflexed, further studies evaluating the relationship between the angle of uterine flexion, the degree of flexion and CSD sizes is needed.
Cesarean scar defect may lead to istmocele which required surgery. Recent literature showed that transvaginal and hystoroscopic surgical approaches are effective [17, 18]. The CSD may be a risk factor for uterine rupture in pregnancy [19]. Clear elucidation of uterine mechanics is key to understanding healing of cesarean incisions [20, 21]. The natural dynamic contraction of the post-delivery uterus is complicated; post-CS uterine incisions and suturing complicate it even further. Importantly, incomplete closure of the uterine wall is hypothesized to be a surgical factor contributing to CSD pathogenesis, as likely is adhesion formation, which played important role in the biomechanical process and postpartum symptoms related to CSD [22, 23, 24]. We hypothesize that physical forces and myometrial contraction in the early months postpartum also play an important role in CSD formation. Roberge et al. [20] concluded that double-layer closure with an unlocked superficial layer excluding the decidua was associated with better uterine scar healing than locked single-layer closure in a 3-arm randomized clinical trial. Most prior studies reported that the retroflexed uterus has much higher rates of CSD occurrence [11, 12, 13]. Our study, although, suggests there was no difference in the rates of CSD among uterus flexion, we can see there is a tendency that the anteflexion uterus is more likely to have a CSD.
In view of previous biomechanical evaluations of postpartum uterine contractions, our findings can also be explained by mechanical analysis of myometrial tissue: the outer myometrium lies mainly in the longitudinal axis; its contraction provides force to the fundal region superior to and the cervix inferior to the cesarean incision while the woman is in a standing or sitting position. These opposing forces may cause separation at the uppermost area of this niche (as the green arrow details in Fig. 2). In terms of the force of gravity on the uterus (blue arrow in Fig. 2), flexion to various degrees exerts forces on the superior border of the niche as the pivot lies in the posterior wall of the retroflexed and anterior wall of the anteflexed uterus. These phenomena contribute to the separation of the niche opening in the anteflexed situation and the power of its closing in the retroflexed situation (as the red arrow details in Fig. 2). We considered gravity to be another force and analysed myometrial function layer by layer, although obviously postpartum women do not stand or sit exclusively.
 Fig. 2.
Fig. 2.Mechanical differences among anteflexed and retroflexed uterus. Outer myometrial contraction generates forces on the fundus superior to the cesarean incision and the cervix inferior to the incision (green arrow). The force of gravity (blue arrow) on the uterus assists in separation of niche opening in the setting of anteflexion and its closing in the setting of retroflexion (red arrow).
To our knowledge, this is the largest retrospective case control study conducted focusing on uterine position changes after vaginal or CS delivery in the setting of singleton pregnancy. As there is a lack of an accepted gold standard for defining uterine position on ultrasonography [15], researchers apply variable definitions and temper investigative approaches in their studies. While our study applied a simple classification of uterine position, more detailed studies are required for a clearer understanding of CSD pathogenesis. Mechanical analyses alone of the postpartum uterus, however, are inadequate. Here, we did not consider forces of the inner myometrium or those of adjacent tissues; neither were different human positions considered. Our findings, however, underscore that uterine position and mechanical forces play vital roles in the development of CSD.
Independent paired TVU prior to 8 weeks gestation and 3–6 months postpartum in women who underwent either vaginal or CS delivery revealed uterine retroflexion in the setting of both delivery modes. CS resulted in significantly more retroflexion than did vaginal delivery. CSD prevalence in postpartum, retroflexed uterus was found to be slightly, but not significantly, lower than in anteflexed uterus. Further detailed research is required to clearly understand post-CS uterine mechanics and is vital to an in-depth comprehension of CSD pathogenesis.
This study revealed that uterus became retroflexed in patients who underwent either CS or vaginal deliveries. CS resulted in a significantly greater proportion of retroflexed uterus than did vaginal delivery. CSD prevalence among women with retroflexed uterus in the postpartum has no difference with those with anteflexed uterus.
HP and FL designed the study. AG collected the clinical data. YY and ZC performed the ultrasonic examination. All authors read and approved the final manuscript.
This study was approved by the Ethics Committee (No. GKLW 2017-126) of the International Peace Maternity and Child Health Hospital (IPMCH, Shanghai, China) on 21 December 2017. All participants provided written, informed consent prior to participation after study methods and objective clarification by a trained physician.
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This study was supported by: the medical engineering cross youth funds from Shanghai Jiao Tong University: YG2017QN38; funds from Education Department of Zhejiang Province; Y202146291.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
