†These authors contributed equally.
Academic Editor: Shigeki Matsubara
Background: To evaluate the feasibility of serum microelements, amino
acids and acyl carnitine levels to predict maternal
complications and adverse infant outcomes in pregnancies
complicated by preeclampsia. Methods: We launched a prospective study
including 81 pregnant patients. Serum microelements, amino acids and acyl
carnitine levels were tested using external calibration technique or
high-performance liquid chromatography tandem mass spectrometry. Concentrations
of metabolites were compared between the preeclampsia and control groups.
Logistic regression models were used to assess the relevance between the
metabolites and pregnancy outcomes in preeclampsia patients without severe
features. Results: Concentrations of some microelements, amino acids and
acyl carnitines were significantly higher or lower in preeclampsia patients.
Women with severe preeclampsia had significantly lower (hexadecanoyl carnitine +
octadecenoyl carnitine)/acetyl carnitine [(C16 + C18:1)/C2] compared to mothers
without severe features. Lower (C16 + C18:1)/C2 was found in women who developed
maternal complications (p = 0.07) or experienced adverse infant outcomes
(p
Preeclampsia (PE) is a multisystem disease occurring in pregnancy that is defined as an elevation of blood pressure with proteinuria or other maternal organ damage after 20 weeks of gestation [1]. PE complicates approximately 6% of pregnancies globally and accounts for more than 10% of maternal death, especially in low-income countries [2, 3]. Previous data indicated that some markers predicted the occurrence of PE and PE-severity, however, the models either have had limited predictive value or could not be replicated in further studies [4, 5].
Some patients with PE may experience complications such as eclampsia or heart failure following conservative management, while other women can deliver at term without adverse events [1]. Due to the unpredictable nature of adverse outcomes in this population, there is no clinical data or laboratory test which has been proved to predict adverse outcomes with high accuracy [6, 7, 8].
Information is lacking on whether the changes in amino acids or acyl carnitines levels occur prior to the occurrence of adverse events in patients with PE. Our study aimed to investigate possible correlations between concentrations of microelement, amino acids and acyl carnitines and clinical outcomes and to expand the search for new biomarkers for prediction of maternal and perinatal outcomes in women with PE.
This prospective cohort study was approved by the medical ethics committee of Hangzhou Women’s Hospital (No. 2018-007-01) and carried out between January 2019 and December 2020. Pregnant women with/without PE during the third trimesters of pregnancy were invited to participate and written informed consent was obtained. The PE group consisted of 49 patients with pregnancy complicated by PE while the other group included 32 normotensive controls who were randomly selected without matching. PE was diagnosed according to ”Obstetrics and Gynaecology”, using the eighth edition diagnostic criteria for China [9]. Patients who had received treatment and then referred to our hospital were also included in our study. Pregnancies with fetal congenital anomalies or stillbirths were excluded.
Data on maternal characteristics including age, gestational age, parity, weight,
height, gestational weight gain and medical history were subsequently collected
on women in both groups. The occurrence of proteinuria and blood pressure
measurements within 48 hours before enrollment in the PE group were also
recorded. Maternal blood was drawn upon enrollment regardless of fasting status
or recent consumption of fatty foods. Collected blood was allowed to stand from
30 to 60 minutes for coagulation stratification. After centrifuging at 3000 rpm
for 10 minutes at room temperature, the supernatant was transferred to a new
centrifuge tube. This tube was centrifuged at 1,2000 rpm for 10 minutes at 4
Data on pregnancy outcomes was collected through hospital medical records.
Maternal outcomes comprised blood pressure measurements, use of antihypertensive
drugs, placental abruption, and progression to severe PE which was diagnosed when
at least one of the following occurred: systolic blood pressure
Before performing analyses of microelements, serum specimens were thawed, vortexed, and centrifuged at 5000 rpm for 5 minutes. Following this they were then analyzed utilizing inductively coupled plasma mass spectrometer NexION 350D (PerkinElmer, Shelton, CT, USA) which was calibrated using an external calibration technique.
Amino acid releasing agents were purchased from Baichen medical laboratory Co.,
Ltd. (Hangzhou, Zhejiang, China), which was used for the pretreatment of dry blood filter
paper to release the bound amino acids for clinical detection of free amino
acids. Amino acid carboxyl groups react with alcohol groups to form esters which
improve the response in mass spectrometry. The stock solutions of internal
standard were prepared in amino acid releasing agent A/water (1:50). After vortex
mixing for 3 minutes and centrifuging at 11000 rpm for 10 minutes,
70
For the analyses of acyl carnitines, a singe 3-mm diameter dry blood spot (1/8
inch) was placed in a well of a U-bottom microstate polystyrene plate (Guangzhou
Fenghua Biological Engineering Co., Ltd, Guangzhou, Guangdong, China) and 100
Analyses of amino acids and acyl carnitines were conducted using an Acquity HPLC chromatographic system coupled to an Acquity TQD tandem-quadrupole mass spectrometer (Waters, Milford, MA, USA). Acquired data were evaluated using TargetLynx software (Waters), by integration of assigned Multiple Reaction Monitoring peaks and normalization using proper internal standards. Personnel data of the groups were concealed from the laboratory inspectors. For the analyses of serum specimens, we quantified 8 trace elements, 28 amino acids or derivatives and 25 acyl carnitine derivatives (Supplementary Appendix 1).
The data were compared using Student’s t/t’ test, Mann-Whitney test, Chi-square, or Fischer exact-probability test as appropriate. First, we compared the concentrations of metabolites in the PE group and control group with metabolites having significant differences between groups being selected for further analysis. Second, we compared the concentrations of the foregoing metabolites in women with severe PE and non-severe PE and compared the concentrations of the metabolites with significant differences in non-severe PE women who developed maternal complications or adverse infant outcomes with those of women who did not develop any adverse outcomes. For metabolites with significant differences in the second analyses, we speculated that the levels of these metabolites might be associated with the severity of PE and would be potential biomarkers for prediction of maternal and perinatal outcomes in women with non-severe PE. Third, the metabolites were inputted into logistic regression models. Receiver operating characteristic (ROC) curves were constructed to assess sensitivity, specificity, and respective AUC with a 95% CI, thus estimating the feasibility of using single significant metabolites in predicting pregnancy outcomes in women with non-severe PE A p value less than 0.05 was considered significant. Statistical analyses were carried out by SPSS version 25.0 (IBM, Armonk, NY, USA).
The demographics of patients in both groups are shown in Table 1. Age and gestational age were not different between the groups. Patients in PE groups had a higher body mass index than the controls, but the variable of gestational weight gain was not different between the groups. Women with PE had significantly higher Cr, Mg, Tyr, Aba, C0, C2, C5:1, C18:1OH, C8/C10 and C16OH/C16 in the blood compared to control patients (Fig. 1). Women with PE had significantly lower Ca, Zn, Aspa, C10, C10/C3, C12/C3, C18/C3, C3/C0, C8/C2, C16/C2 and (C16 + C18:1)/C2 in the blood compared to control mothers. Because some women with PE had received magnesium sulphate before enrollment, Mg and Ca were excluded from further analysis [10].
| Preeclampsia (n = 49) | Controls (n = 32) | p | ||
| Age, year | 30.0 |
28.8 |
0.43 | |
| Pre-pregnancy body mass index, kg/m |
23.9 |
20.2 |
||
| Gestational age, weeks | ||||
| 26 (53.1) | 17 (53.1) | 0.73 | ||
| 34–37 | 14 (28.6) | 11 (34.4) | ||
| 9 (18.4) | 4 (12.5) | |||
| Gestational weight gain, kg | 14.0 |
14.3 |
0.83 | |
| Number of live fetuses | ||||
| 1 | 43 (87.8) | 30 (93.8) | 0.47 | |
| 2 | 6 (12.2) | 2 (6.3) | ||
| Parity | ||||
| 0 | 37 (75.5) | 30 (93.8) | 0.03 | |
| 1 | 12 (24.5) | 2 (6.2) | ||
| Data are n (%), mean (SD). | ||||
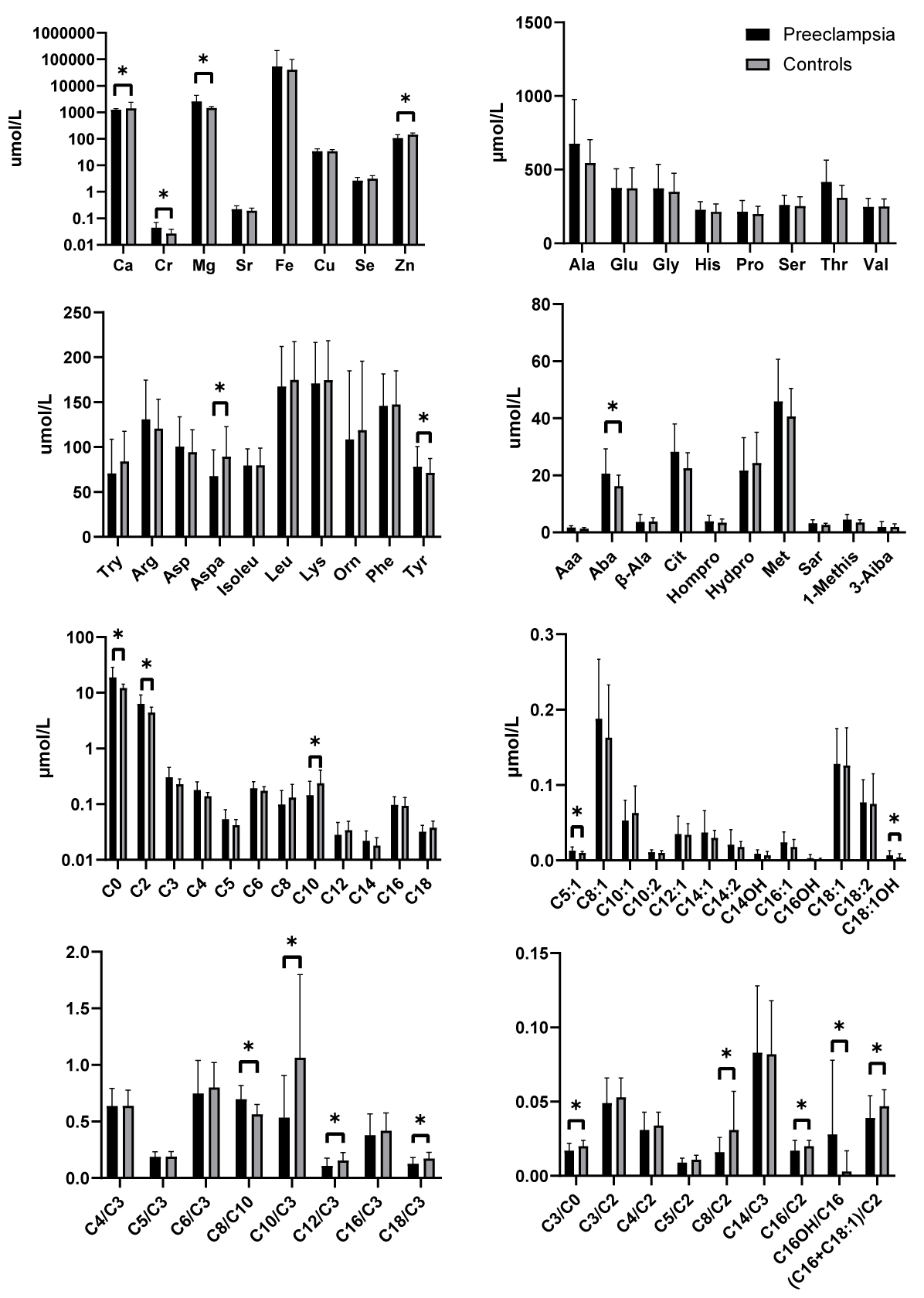 Fig. 1.
Fig. 1.
The levels of microelement, amino acids and acylcarnitine in
women who developed preeclampsia and controls (*p
Forty nine patients were stratified by severity of PE with 26 of them being classified as severe PE. Except for blood pressure and proteinuria, no significant differences could be found in other variables between women with different PE severity (Table 2). Compared to mothers with non-severe PE, women with severe PE had significantly higher levels of C2, C18:1OH and C16OH/C16 in the blood, but significantly lower ratio of (C16 + C18:1)/C2 (Fig. 2).
| Non-severe (n = 23) | Severe (n = 26) | p | ||
| Age, year | 30.0 |
30.0 |
0.98 | |
| Pre-pregnancy body mass index, kg/m |
24.9 |
22.9 |
0.13 | |
| Gestational age, weeks | ||||
| 10 (43.5) | 16 (61.5) | 0.37 | ||
| 34–37 | 7 (30.4) | 7 (26.9) | ||
| 6 (26.1) | 3 (11.5) | |||
| Gestational weight gain, kg | 14.1 |
14.0 |
0.93 | |
| Number of live fetuses | ||||
| 1 | 20 (87.0) | 23 (88.5) | 0.87 | |
| 2 | 3 (13.0) | 3 (11.5) | ||
| Parity | ||||
| 0 | 18 (78.3) | 19 (73.1) | 0.67 | |
| 1 | 5 (21.7) | 7 (26.9) | ||
| Interval from last pregnancy, year | ||||
| 1 (20.0) | 1 (14.3) | 1.00 | ||
| 5–10 | 3 (60.0) | 5 (71.4) | ||
| 1 (20.0) | 1 (14.3) | |||
| History of preeclampsia | ||||
| No | 20 (87.0) | 25 (96.2) | 0.33 | |
| Yes | 3 (13.0) | 1 (3.8) | ||
| Chronic hypertension | ||||
| No | 21 (91.3) | 25 (96.2) | 0.59 | |
| Yes | 2 (8.7) | 1 (3.8) | ||
| Gestational diabetes | ||||
| No | 17 (73.9) | 22 (84.6) | 0.48 | |
| Yes | 6 (26.1) | 4 (15.4) | ||
| Highest systolic blood pressure, mmHg | 145.7 |
160.6 |
||
| Mean systolic blood pressure, mmHg | 138.5 |
153.4 |
||
| Highest diastolic blood pressure, mmHg | 94.0 |
104.7 |
||
| Mean diastolic blood pressure, mmHg | 88.3 |
99.9 |
||
| Protein per 24 hour urine collection, mg | 1126.8 |
4065.1 |
||
| Data are n (%), mean (SD). | ||||
 Fig. 2.
Fig. 2.
The levels of microelement, amino acids and acylcarnitine in
patients stratified by severity of preeclampsia (*p
In 23 patients with non-severe PE, neither placental abruption, stillbirth or
neonatal death before hospital discharge was reported. Blood pressure exceeds the
limits established in five patients of whom four received intravenous
antihypertensive medication. Seven patients progressed to severe PE and 10 babies
were admitted to intensive care unit. One case of an Apgar score
In patients with non-severe PE, the concentrations of C2, C18:1OH and (C16 +
C18:1)/C2 were compared between women who developed maternal complications or
adverse infant outcomes and those who did not. (C16 + C18:1)/C2 was found to be
lower in women who developed maternal complications (p = 0.07) or
adverse infant outcomes (p
 Fig. 3.
Fig. 3.
The levels of acyl carnitine in women who developed maternal
complications or adverse infant outcomes compared with those who did not in
patients with non-severe preeclampsia (*p
 Fig. 4.
Fig. 4.ROC curves for diagnostic accuracy of (C16 + C18:1)/C2 ratio for the outcomes. (A) Prediction of maternal complications. (B) Prediction of adverse infant outcomes.
Our hypothesis was that increased or decreased concentration of some metabolites may play a role in the aggravation of PE and concentrations of these metabolites may have changed prior to the aggravation of symptoms in women with PE. In our study, we found PE was associated with abnormal concentrations of some microelements, amino acids and acyl carnitines. Concentrations of four metabolites were associated with the severity of PE and (C16 + C18:1)/C2 seemed to be a potential biomarker in the prediction of pregnancy outcomes in non-severe PE patients.
This study showed a significant decrease in the serum Zn concentration of PE patients compared to controls as has been observed in previous publications [11, 12, 13, 14, 15]. Açikgoz et al. [13] reported a negative correlation between angiotensin converting enzyme activity and Zn concentration and demonstrated that Zn deficiency in the placenta could lead to superoxide dismutase insufficiency resulting in an oxidative stress which may play a role in the pathophysiological processes of PE. We also found higher Cu and lower Se concentrations in our PE group, though neither reached statistical significance as demonstrated in previous publications [15, 16, 17, 18].
Our study found lower levels of Aspa in PE patients compared to the control group, which was compatible with the result of a previous study [19]. Though the correlation between lower level of Aspa and PE development remains unclear, our results suggest Aspa deficiency may be a risk factor during the aggravation of PE. We also observed significant higher Tyr levels in PE patients with the results being consistent with those of a recent study [20]. We speculate that higher Tyr levels may be associated with the production of soluble fms-like tyrosine kinase 1, a potent antiangiogenic factor known to contribute to the maternal syndrome [21].
PE is related to lipid metabolism disorders, including fatty acid oxidation
metabolism [22]. Metabolic abnormality of fatty acid in the placenta has been
proved to release toxic metabolites which cause damage to vascular endothelium
[23]. In pregnancy complicated by PE, increase of
Previous studies have investigated the predictive values of acyl carnitines for PE in different trimesters of pregnancy [38, 39]. Koster et al. [40] analyzed 24 metabolites in maternal serum in early pregnancies between patients with PE and controls, Twelve acyl carnitines were significantly different in early-onset PE patients, with effect levels ranging from an 18% decrease to a 29% increase. Liu et al. [20] analyzed 49 types of amino acids or acyl carnitines in maternal blood and compared the levels between women with PE and healthy pregnancies with the majority of them being significantly higher in pregnancies with PE. Previous studies as well as the current study report different metabolomic biomarkers for PE, which may partially be resulted from the differences in the sample material used, disease stage and metabolomics protocol and analysis utilized in the studies.
We believe that this is the first study to investigate (C16 + C18:1)/C2 in the prediction of pregnancy outcomes in non-severe PE patients. Though the underlying mechanism is still unknown, abnormal (C16 + C18:1)/C2 ratio seem to affect the activity of the heart and muscle carnitine palmitoyl transferase 2, which incapacitates the acyl-carnitine-mediated mitochondrial oxidation of long-chain fatty acids, causing severe cardiac hypertrophic remodeling, dilation, and heart failure [41].
Our study has some limitations. First, we collected samples from pregnant women from 28 weeks to full term and gestational age was comparable between the groups. However, as stated in previous articles, levels of the metabolites swing across the gestational age spectrum, which might have affected our results [42, 43, 44, 45, 46]. Second, we did not control for fasting or recent consumption of fatty foods or other dietary variables when collecting blood samples. It is suggested that these confounding factors may influence the levels of the metabolites, thus reducing the credibility of the results [47, 48, 49]. Third, the number of patients included was small and some outcomes could not be calculated. Concerning the foregoing limitations, it is uncertain whether (C16 + C18:1)/C2 ratio could be used as a biomarker to predict adverse events in non-severe PE patients. Future studies are required to explain the underlying molecular mechanism between (C16 + C18:1)/C2 ratio and PE.
Maternal levels of microelements, amino acids and acyl carnitines were associated with PE. (C16 + C18:1)/C2 seemed to be a potential biomarker in the prediction of maternal complications and adverse infant outcomes in non-severe PE patients. Further studies are needed to validate its potential usefulness.
MZ, HLW and YC designed the research study. MZ and LMY performed the research. CHW and YC provided help and advice on the experiments. HLW and TFL analyzed the data. MZ, HLW and YC wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
This study was approved by the medical ethics committee of Hangzhou Women’s Hospital (No. 2018-007-01). All participants in this study had provided written informed consent.
Thanks to all the peer reviewers for their opinions and suggestions.
The report was supported by Zhejiang basic public welfare research project (LGF19H040007 to Yun Chai).
The authors declare no conflict of interest.
