†These authors contributed equally.
Academic Editor: Johannes Ott
Background: The abnormal course of the umbilical vein and the absence or displacement of the portal vein or ductus venosus may suggest the presence of other shunts. In recent years, an in utero classification of umbilical-portal-systemic venous shunts (UPSVS) has been introduced. This study aims to report our experience with the prenatal diagnosis of UPSVS and to evaluate the perinatal outcomes of fetuses with different types of UPSVS. Methods: This was a retrospective study of fetuses prenatally diagnosed with UPSVS between January 2005 and September 2020 at Asan Medical Center. UPSVS was prenatally classified into three types according to the anatomical origin of the shunt: type I, umbilical-systemic shunt; type II, ductus venosus-systemic shunt; and type III, portal-systemic shunt. Type III was further divided into two subtypes: type IIIa, intrahepatic shunt; type IIIb, extrahepatic shunt. Postnatal ultrasonography or abdominal computed tomography (CT) was performed to confirm UPSVS. Results: Out of 18 fetuses prenatally diagnosed with UPSVS, four were excluded. All four patients were confirmed to have portal vein variations without a shunt. The median gestational age at the diagnosis of the remaining 14 fetuses was 30.3 weeks (range, 23.1–37.3). The median gestational age at birth was 37.2 weeks (range, 31.0–39.3), and the median birth weight was 2105 g (range, 1350–2870), including nine (64%) who had body weights below the 10th percentile. Eleven patients (79%) had combined anomalies; cardiomegaly and fetal hydrops were observed in eight (57%) and two (14%) patients, respectively. Among the 11 patients who underwent chromosomal analysis, three had abnormal results. Two infants with abnormal karyotypes died. The most common type was type I (8, 57%), followed by type IIIa (4, 29%), type II (1, 7%), and type IIIb (1, 7%). In type I, all but one patient had structural anomalies; five (63%) showed cardiomegaly and five (63%) were associated with growth restriction. Nevertheless, half of the patients survived without major anomalies. In type IIIa, three (75%) patients were associated with growth restriction, but they showed catch-up growth after birth. All patients had mild transient hyperammonemia. One type II patient was associated with cardiomegaly, fetal hydrops, and an abnormal karyotype. One type IIIb patient had multiple anomalies and an abnormal karyotype. No surgical correction was required because of the UPSVS. Conclusions: Prenatal diagnosis of UPSVS is feasible. When an abnormal course of the umbilical or portal vein is detected on the transverse abdominal view, UPSVS should be suspected. UPSVS is commonly associated with structural anomalies, chromosomal anomalies, growth restriction, and cardiomegaly. UPSVS without associated anomalies may have a favorable prognosis. Our study can help predict the prognosis of UPSVS and assist in prenatal counseling.
In a fetal assessment by ultrasonography, an abnormal course of the umbilical vein (UV) at the transverse abdominal view could be indicative of an abnormal fetal venous system. The abnormal course of the UV and the absence or displacement of the portal vein (PV) or ductus venosus (DV) may suggest the presence of other shunts [1]. This condition is rare and has been reported in the context of an absent DV or by using a postnatal dichotomous classification that does not reflect the importance of the DV [2, 3].
In 2016, Achiron et al. [4] suggested an in utero classification of umbilical-portal-systemic venous shunt (UPSVS), which include three types: type I, umbilical-systemic shunt (USS); type II, DV-systemic shunt (DVSS); and type III, portal-systemic shunt (PSS). Furthermore, PSS consists of two subtypes: type IIIa, intrahepatic portal-systemic shunt (IHPSS) and type IIIb, extrahepatic porta-systemic shunt (EHPSS). However, only one study to date has reported the application of this new classification system. In that study, half of the study population was terminated [5]; therefore, it is difficult to describe the characteristics of each UPSVS type based on the available data.
The aim of this study was to report our experience on the prenatal diagnosis of UPSVS according to the in-utero classification discussed and to demonstrate the perinatal outcomes of fetuses according to the type.
We retrospectively reviewed fetuses prenatally diagnosed with UPSVS between
January 2005 and September 2020 at Asan Medical Center, Seoul, Republic of Korea.
This study was approved by the institutional review board of our institution (No.
2021-0277). All prenatal ultrasound examinations underwent an anatomical survey,
including fetal echocardiography with Accuvix XQ, V10, A30, or WS80A (Samsung
Medison Co., Ltd., Seoul, Republic of Korea), Aloka ProSound
We evaluated the transverse abdominal view to confirm the integrity of the umbilical-portal-DV complex and classified the cases according to UPSVS type. The UPSVS types are described in Table 1, and schematic diagrams of each type are shown in Fig. 1. One expert in maternal-fetal medicine reviewed all ultrasonographic images with medical reports and classified the cases according to UPSVS type.
 Fig. 1.
Fig. 1.Schematic diagrams of umbilical-portal-systemic venous shunt. Shunts are indicated as arrows. UA, umbilical artery; UV, umbilical vein; IVC, inferior vena cava; RA, right atrium; RV, right ventricle; LA, left atrium; LV, left ventricle; LPV, left portal vein; MPV, main portal vein; RPV, right portal vein; HV, hepatic vein; DV, ductus venosus.
| Type | Name | Description |
| I | USS | Agenesis of both the DV and the left branch of portal vein results in a normal IHPVS failing to form. Umbilical vein is directly connected to the systemic circulation. |
| II | DVSS | A short DV is connected to the systemic vein with an intact umbilical-portal-DV complex. Mainly, the location of DV is different or shorter than usual. |
| III | PSS | |
| IIIa | IHPSS | The shunt between IHPVS and the hepatic veins is present. |
| IIIb | EHPSS | The shunt between the portal system and systemic veins is present. |
| UPSVS, umbilical-portal-systemic venous shunt; USS, umbilical-systemic shunt; DV, ductus venosus; IHPVS, intrahepatic portal venous system; DVSS, ductus venosus-systemic shunt; PSS, portal-systemic shunt; IHPSS, intrahepatic portal-systemic shunt; EHPSS, extrahepatic portal-systemic shunt. | ||
Prenatal data included maternal age, gestational age (GA) at diagnosis, chromosome analysis, associated anomaly, fetal hydrops, fetal cardiomegaly, fetal growth restriction, type of UPSVS, GA at delivery, and birth weight. Fetal growth restriction was defined as birth weight below the 10th percentile based on Asian standards for fetal growth [6]. Fetal cardiomegaly was defined as a cardiothoracic (C/T) area ratio or a C/T circumference ratio greater than two standard deviations [7]. Fetal hydrops is a fetal condition defined as abnormal accumulation of fluid in two or more spaces, which may include ascites, pleural effusion, pericardial effusion, and skin edema.
All babies were examined by neonatal specialists and had postnatal diagnosis of UPSVS, which was further confirmed by abdominal ultrasound or computed tomographic (CT) angiography. Postnatal data included clinical and biochemical findings caused by UPSVS, any indication for surgery or intervention for UPSVS, and duration of follow-up. Structural abnormalities found after birth were also included.
The data were presented as medians with ranges or as numbers with percentages.
Among the 18 patients prenatally suspected with UPSVS, 14 were confirmed with UPSVS postnatally and four were confirmed with PV variants. The most common type of USPVS was type I (n = 8, 57%), followed by type IIIa (n = 4, 29%), type II (n = 1, 7%), and type IIIb (n = 1, 7%). The clinical characteristics of the study population are summarized in Table 2. Cardiomegaly and fetal hydrops were observed in eight (57%) and two (14%) patients, respectively. Among the 11 patients who underwent chromosomal analysis, three had abnormal results: trisomy 18, 3p deletion syndrome, and unbalanced (X;22) translocation. Two of the patients died during the infant period. Table 3 presents the detailed outcomes of all the patients diagnosed with UPSVS.
| Characteristics | ||
| Maternal age (years) | 35.5 (28–40) | |
| GA at diagnosis (weeks) | 30.2 (23.1–37.3) | |
| GA at birth (weeks) | 37.2 (31.0–39.3) | |
| Birth weight (g) | 2105 (1350–2870) | |
| 9/14 (64%) | ||
| Abnormal karyotype (n, %) | 3/11 (27%) | |
| Trisomy 18 | 1 | |
| 3p deletion | 1 | |
| Unbalanced (X;22) translocation | 1 | |
| Overall survival rate at 28 days after birth | 100% | |
| Associated anomaly (n, %) | 11/14 (79%) | |
| UPSVS, umbilical-portal-systemic venous shunt; GA, gestational age. Data are presented as median (range) or number (%). | ||
| Case | UPSVS type | Associated anomaly | Karyotype | Fetal hydrops | Cardiomegaly | Growth restriction | GAD (weeks) | BW (g) | Follow-up period |
| 1 | I | minor | Normal | – | + | – | 38.0 | 2800 | 3 m |
| 2 | I | None | Normal | – | + | +* | 38.3 | 2040 | 6 y |
| 3 | I | Multiple anomalies | Normal | + | – | +* | 37.1 | 1980 | 2 m |
| 4 | I | Isolated extracardiac | – | – | + | – | 39.1 | 2870 | 4 y |
| 5 | I | Multiple anomalies | Trisomy 18 | – | – | + | 38.6 | 2580 | Died at 7 m |
| 6 | I | Multiple anomalies | Normal | – | – | +* | 37.6 | 1910 | 2 y 7 m |
| 7 | I | Isolated cardiac | Normal | – | + | – | 33.3 | 2560 | 2 y 7 m |
| 8 | I | Multiple anomalies | Normal | – | + | +* | 39.4 | 2520 | 1 y 6 m |
| 9 | II | Multiple anomalies | 3p deletion | + | + | – | 31.0 | 2170 | 1 y 5 m |
| 10 | IIIa | Isolated cardiac | Normal | – | + | +* | 36.6 | 1793 | 1 y 2 m |
| 11 | IIIa | None | – | – | – | – | 36.6 | 2490 | 2 y 3 m |
| 12 | IIIa | None | Normal | – | – | +* | 34.6 | 1550 | 7 m |
| 13 | IIIa | Minor | – | – | + | +* | 37.4 | 1900 | 4 m |
| 14 | IIIb | Isolated cardiac | t(X;22) | – | – | +* | 32.4 | 1350 | Died at 3 m |
| UPSVS, umbilical-portal-systemic venous shunt; GAD, gestational age at delivery;
BW, birth weight; t(X;22), unbalanced (X;22) translocation; m, months; y, years; –, absent finding; +, present finding.
* below 3rd percentile birth weight. | |||||||||
There were eight cases of type I UPSVS. These cases did not show any DV, and the UV was connected to the systemic vein. In six cases (cases 1, 4–8), the UV directly drained into the right atrium (Fig. 2). In two cases (cases 2 and 3), the UV drained into the inferior vena cava (IVC). All but one (7/8, 88%) were associated with other structural abnormalities: two cases with VACTERL association (cases 6 and 8), which included three or more combinations of vertebral defects (V), anal atresia (A), cardiac abnormalities (C), tracheoesophageal fistula (TE), renal abnormalities (R), and limb defect (L). And one case with Cornelia de Lange syndrome (case 3), which shows distinctive facial characteristics and growth delays [8]. The patient with trisomy 18 died at the age of seven months because of respiratory failure due to tracheomalacia (case 5). Cardiomegaly was observed in five fetuses, three of which resolved. The median GA at birth was 38.1 weeks (range, 33.3–39.4) and the median birth weight was 2540 g (range, 1910–2870). Five neonates had birth weights below the 10th percentile, and four of them were below the 3rd percentile.
 Fig. 2.
Fig. 2.Two-dimensional color Doppler image of type I umbilical-portal-systemic venous shunt in case 7. Umbilical vein directly drains into the right atrium without going through the ductus venosus. RA, right atrium.
We found one case of type II UPSVS (case 9). The fetus had a short DV that drained into the IVC, but other structures of the umbilical-portal-DV complex were intact. The fetus showed cardiomegaly and fetal hydrops with severe skin edema and pleural effusion (Fig. 3). This fetus was confirmed to have a 3p deletion. The fetal hydrops resolved after birth. At the time of writing, the patient was 17 months old and had developmental delay.
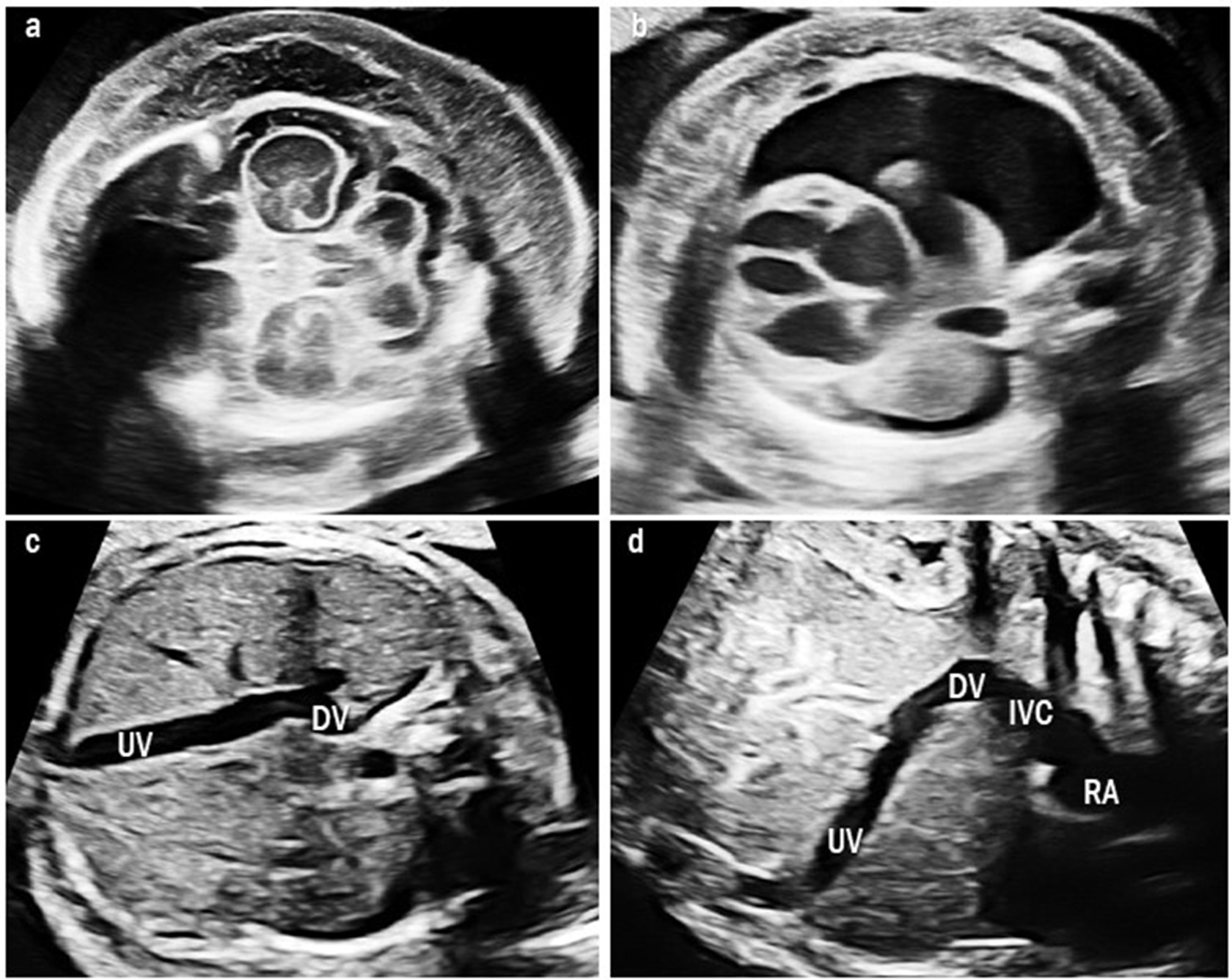 Fig. 3.
Fig. 3.Ultrasound images of type II umbilical-portal-systemic venous shunt in case 9. The fetus showed severe fetal hydrops: (a) severe skin edema and (b) pleural effusion. (c) Transverse abdominal image shows the UV connected to the DV and it drains at the lower site than usual. (d) Sagittal image shows a short DV draining into the IVC below the diaphragm. UV, umbilical vein; DV, ductus venosus; IVC, inferior vena cava; RA, right atrium.
Four cases were classified as type IIIa UPSVS. They showed an abnormal connection between the PV and hepatic vein, which was observed as a tortuous and engorged vessel in the fetal liver (Fig. 4). However, the umbilical-portal-DV complex remained intact. Among these cases, three (75%) had birth weights below the 10th percentile and all had transient mild hyperammonemia. More importantly, none of them required shunt ligation surgery or liver transplantation.
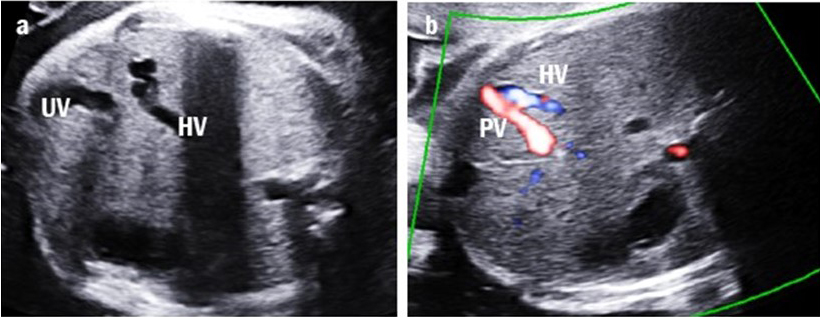 Fig. 4.
Fig. 4.Intrahepatic portal-systemic shunt in case 11. (a) The tortuous and engorged hepatic vein (HV) is shown. (b) The intrahepatic portal vein (PV) is connected to the HV.
One case of type IIIb UPSVS was identified (case 14). Prenatal ultrasonography revealed an abnormal tortuous vessel communicating between the PV and IVC (Fig. 5). Due to this shunting flow, the IVC became enlarged; otherwise, the umbilical-portal-DV complex was intact. The fetus was below the 3rd percentile and had both cardiac and extra-cardiac anomalies. Additionally, the karyotype results showed an unbalanced (X;22) translocation. The neonate suffered from bronchial stenosis and died due to pulmonary hypertension and heart failure at the age of three months.
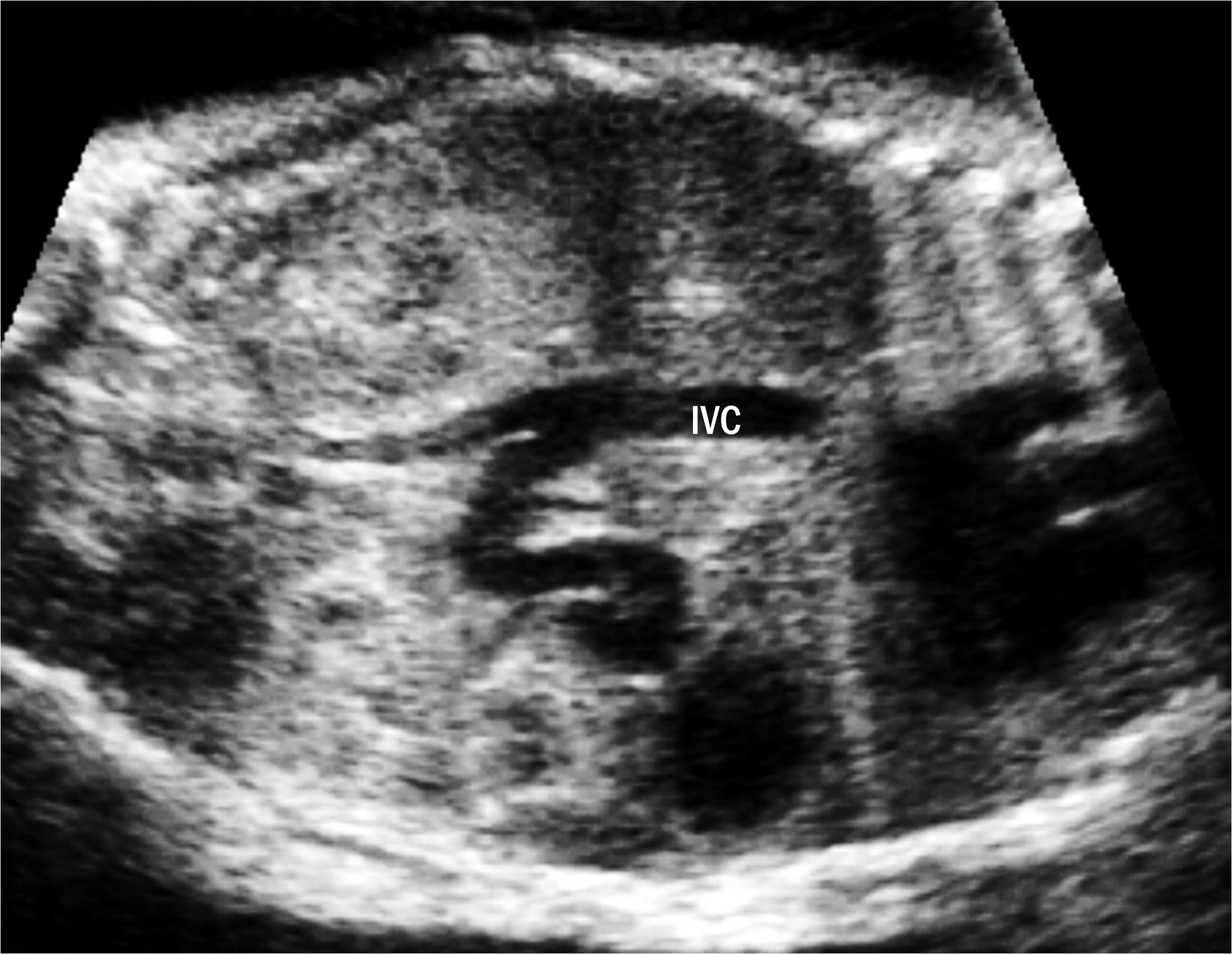 Fig. 5.
Fig. 5.Extrahepatic portal-systemic shunt in case 14. The portal vein (PV) is connected to the inferior vena cava (IVC).
Four cases were confirmed with PV variants on postnatal abdominal sonography. In the prenatal period, the connection between the left PV and the UV was not clear. However, the DV was observed and there was no evidence of PSS. Thus, we could not classify these cases as any type of UPSVS. Furthermore, postnatal ultrasonography showed a PV variant, which was a left PV arising from a right PV or a main PV. Although all patients had associated anomalies, there were no cases of cardiomegaly, and there was one case of growth restriction (Table 4).
| Case | Associated anomaly | Karyotype | Fetal hydrops | Cardiomegaly | Growth restriction | GAD (weeks) | BW (g) | Follow-up period |
| 15 | Isolated extracardiac | Normal | – | – | + | 38.3 | 2067 | 7 m |
| 16 | Isolated cardiac | Normal | – | – | – | 40.0 | 3230 | 4 y 2 m |
| 17 | Multiple anomalies | Normal | – | – | – | 35.4 | 2800 | 2 y 5 m |
| 18 | Multiple anomalies | Normal | – | – | – | 38.0 | 3010 | 11 m |
| GAD, gestational age at delivery; BW, birth weight; m, months; y, years; –, absent finding; +, present finding. | ||||||||
According to our study, prenatal diagnosis and classification of UPSVS are feasible. We introduced 14 cases of UPSVS based on the new classification. These cases were mainly associated with growth restriction and structural abnormalities, and some were accompanied by abnormal chromosomal results. In patients with type I, growth restriction and cardiomegaly are common. If these patients have no other structural abnormalities, the fetus can live well. Nevertheless, accompanying structural anomalies and chromosomal abnormalities may still appear for some; in such cases, targeted ultrasound and chromosomal studies should be recommended.
The previous classification was used without considering the DV. On the other hand, the new in utero UPSVS classification system was based on the embryological-anatomical origin of the shunt. As such, abnormalities in the fetal venous system can be diagnosed by characterizing the prenatal blood flow system.
In South Korea, a study on congenital portosystemic shunts was reported in 2013 [9]. The authors categorized the cases according to the classification suggested by Park et al. in 1990 [10]. Among the six cases included, shunts were diagnosed prenatally in only two cases. The four other cases showed a prominent hepatic vein, abnormal intrahepatic tubular structure, cardiomegaly, or fetal growth restriction. Compared to this study, our study shows that all UPSVS cases were diagnosed before birth and were categorized using the new classification of UPSVS discussed.
Abnormalities associated with the fetal venous system are relevant to fetal growth restriction. In previous reports, the overall percentage of fetal growth restriction observed in patients with UPSVS was 39.5%, with more than 50% reported as types I and IIIa [3]. In this study, 64% of the cases were associated with fetal growth restriction. When comparing the frequency of growth restriction by UPSVS types, type IIIa was the most common with 75% (3/4), which was followed by type I with 63% (5/8). Overall, 55% (5/9) showed catch-up growth after birth. Since there were many cases associated with multiple anomalies, the catch-up growth rate was lower compared to most small for gestational age children. The fetus without major anomalies can grow well after birth due to changes in the portal system. According to a previous study, severe fetal growth restriction resulted in increased DV flow and reduced umbilical vein flow to the liver. Decreased hepatic flow may induce fetal growth restriction as a result [11]. They suggested that the diameter of the right portal vein could be a reliable ultrasound marker to predict fetal growth restriction. Since the UV drains directly into the systemic blood flow in type I UPSVS, there is decreased blood flow in the liver. Consequently, growth restriction may occur in type I UPSVS. Likewise, PSS reduces blood flow to the liver parenchyma, affecting the growth process.
Cardiomegaly was observed in eight fetuses (57%). It resolved in four of these fetuses before birth and in three infants at one week, 12 months, and 31 months after birth, respectively. At the time of writing, the remaining patient still had right ventricular hypertrophy due to associated cardiac anomalies. In the absence or disposition of the DV, cardiomegaly can occur because of the dysregulation of venous return to the heart [12]. In this study, in the absence of congenital heart disease, cardiomegaly spontaneously improved in most cases.
Previous studies have reported that type I UPSVS has the poorest prognosis with the lowest rates of live birth and postnatal survival [4, 5]. In these two studies, 10 out of 13 cases were terminated due to complete absence of an IHPVS. Therefore, these studies were limited in their ability to evaluate perinatal outcomes. In the present study, half of the type I cases were alive and doing well without major anomalies at the time of writing.
There were three cases of chromosomal abnormalities observed in this study that were not reported in any previous studies: trisomy 18, 3p deletion, and unbalanced (X;22) translocation. Several chromosomal anomalies have been reported in cases of abnormal fetal venous systems. Among these, trisomy 21 was the most common karyotype anomaly and was mainly associated with type II UPSVS [4, 5]. Before the introduction of in-utero classification, a study on 19 fetuses with absent DV and umbilical venous drainage in the portal sinus reported two cases of 13p deletion and 4p deletion. The chromosomal abnormalities observed in the three cases in this study were characterized by multiple structural anomalies. Trisomy 18 is characterized by an abnormal central nervous system, congenital heart disease, and limb anomaly [13]. Microcephaly and congenital heart disease were found in the 3p deletion. Unbalanced (X;22) translocation could have similar features as DiGeorge syndrome [14]. There could be UPSVS in these diseases showing multiple anomalies, but it may not have been detected previously due to low level of awareness about UPSVS.
In this study, four cases of PV variation were identified. In the transverse abdominal view, UV insertion and connection of the PV were intact, but the left PV was not identified. The DV was found to be intact, and another right PV course was also observed. Based on our experience, PV variants can be prenatally mistaken for UPSVS. Since the PV was too thin to be evaluated even after birth, it took several months to diagnose the PV variant. We found that an intact umbilical-portal-DV complex and the absence of a portal-systemic shunt could be the points of differential diagnosis between UPSVS and PV variants. Although they had combined anomalies, all four cases were alive at the time of writing. Considering these, PV variation may not be clinically significant in the absence of liver disease.
In conclusion, although UPSVS is a rare fetal disease, prenatal diagnosis of UPSVS is feasible. When an abnormal course of the UV or PV is detected on the transverse abdominal view, UPSVS should be suspected. According to our study, type I and IIIa UPSVS were common. Furthermore, UPSVS is commonly associated with fetal growth restriction, cardiomegaly, structural anomalies, and chromosomal anomalies. UPSVS without other conditions may notably have favorable prognosis. This study was limited by the inability to evaluate the perinatal outcomes of all types due to the small number of cases. Nevertheless, our study introduced additional information about UPSVS and can both help predict the prognosis of UPSVS and assist in prenatal counseling.
JC—extraction and drafting of the manuscript; JK—extraction and drafting of the manuscript; ML—manuscript revision; HW— manuscript revision. All authors read and approved the final manuscript.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Asan Medical Center Institutional Review Board (approval number: 2021-0277).
Thanks to all the peer reviewers for their opinions and suggestion.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
