Academic Editor: Michael H. Dahan
Background: Cystic adenomyosis (CA) is a rare form of adenomyosis. Case: We present for the first time secondary CA in a young woman with increased menstrual volume as the main clinical manifestation. A 23-year-old woman with a history of two uterine surgeries was hospitalized with increased menstrual flow volume and no dysmenorrhea or other discomfort. Ultrasound resulted in misdiagnosis as myoma of the uterus. She underwent laparoscopic surgery for adenomyoma excision with levonorgestrel-releasing intrauterine system (LNG-IUS) placement. During surgery, the lesions were completely removed under ultrasound guidance, and a specimen bag was used to reduce residual lesions in the abdominal cavity. She was postoperative treated with gonadotropin-releasing hormone analog (GnRH-a). Surgical findings and postoperative pathology confirmed CA. She has been followed up for 2 years without recurrence. Conclusions: A CA diagnosis should be considered for clinical manifestations of increased menstrual volume and dysmenorrhea in young patients with previous uterine surgery histories. Magnetic resonance imaging (MRI) is very important for CA diagnosis. Conservative surgery along with GnRH-a and LNG-IUS therapy can effectively prevent recurrence.
Adenomyosis is a common benign gynecologic disease characterized by invasion of
the endometrial matrix or glands into the myometrium [1]. The disease may be focal
or diffuse [2] and may appear as small cystic spaces, usually
Consistent with the treatment principle for adenomyosis, the treatment principle for CA is to completely remove the lesion, promote fertility and prevent recurrence [6]. Complete resection of the adenomyoma and restoration of the normal shape of the uterus can prevent recurrence [7]. According to the location and scope of the lesion, different surgical methods can be selected. According to whether the uterus is retained, surgical intervention can involve conservative surgery or total hysterectomy [8]. CA is more common in women of childbearing age, so it is important to preserve fertility. This disease is a special type of adenomyosis, and there may be residual ectopic endometrium after surgery. Therefore, it is particularly important to take appropriate measures to prevent recurrence. We report for the first time a rare case of a CA secondary to uterine surgery in a 23-year-old woman. Her clinical manifestations were primarily increased menstrual volume without the typical symptom of dysmenorrhea. The patient was successfully treated by the use of conservative surgery combined with gonadotropin-releasing hormone analog (GnRH-a) and a levonorgestrel-releasing intrauterine system (LNG-IUS) for the first time, and no recurrence was found during the 2-year follow-up.
A 20-year-old young woman with no history of pregnancy underwent hysteroscopic submucosal myoma resection. Myoma recurrence was found on reexamination 6 months after surgery. Her menstrual volume had slightly increased, and she had painful menstruation. Since then, there had been a small amount of irregular vaginal bleeding and occasional abdominal distension.
At 21 years old, her ultrasound showed a 4.3
 Fig. 1.
Fig. 1.Ultrasound tomographs of the patient. (A) At the age of 21, the patient’s first ultrasound examination showed a mass
with mixed echogenicity in the uterine cavity of 2.0
At the age of 23, the woman was hospitalized for four months due to increased
menstrual flow. Before admission, her
menstrual volume had increased over the past 4 months to approximately 1.5 times
the original menstrual volume. She had no dysmenorrhea or changes in the
menstrual cycle or menstrual period. Ultrasound demonstrated two hypoechoic
nodules in the posterior wall of the uterus, 4.3
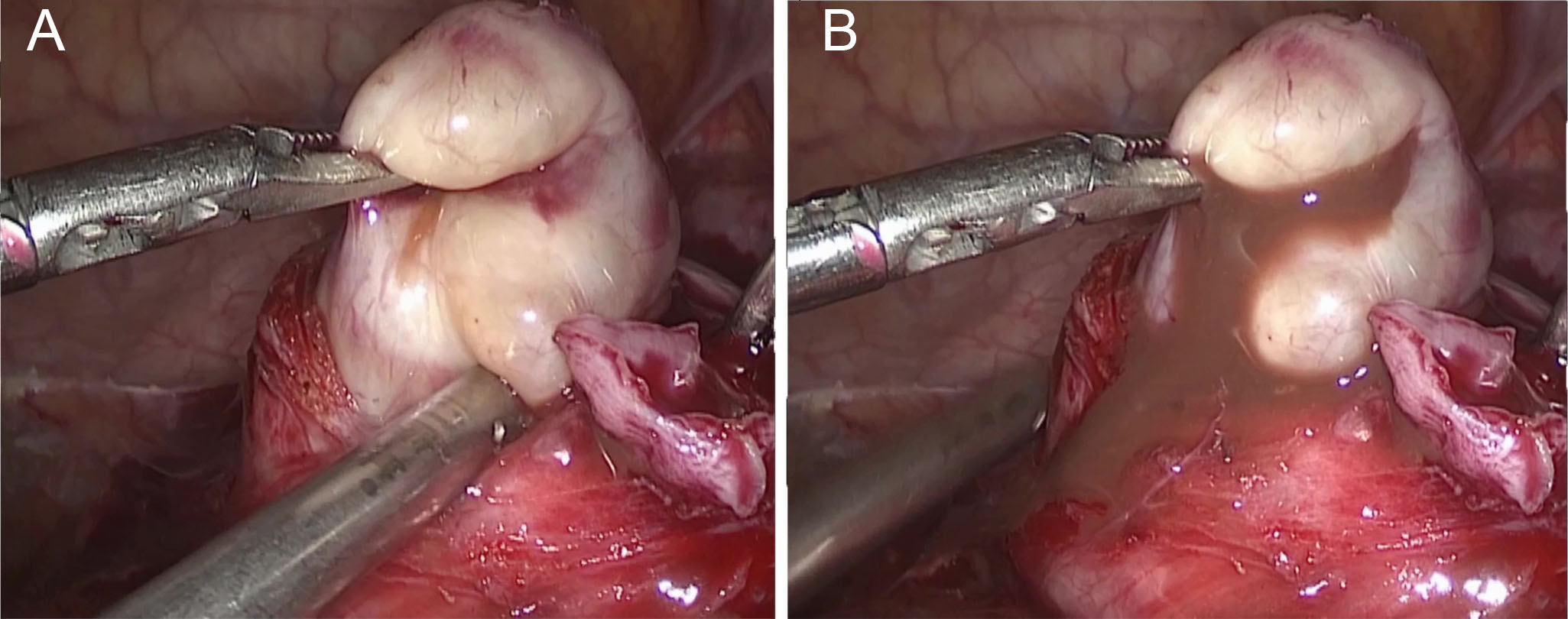 Fig. 2.
Fig. 2.(A) On the left side of the uterus, there was a projection of adenomyomatous material with an irregular, lobulated shape and a diameter of approximately 8 cm. (B) When the cyst roof was opened, chocolate-colored fluid flowed out of the lesion.
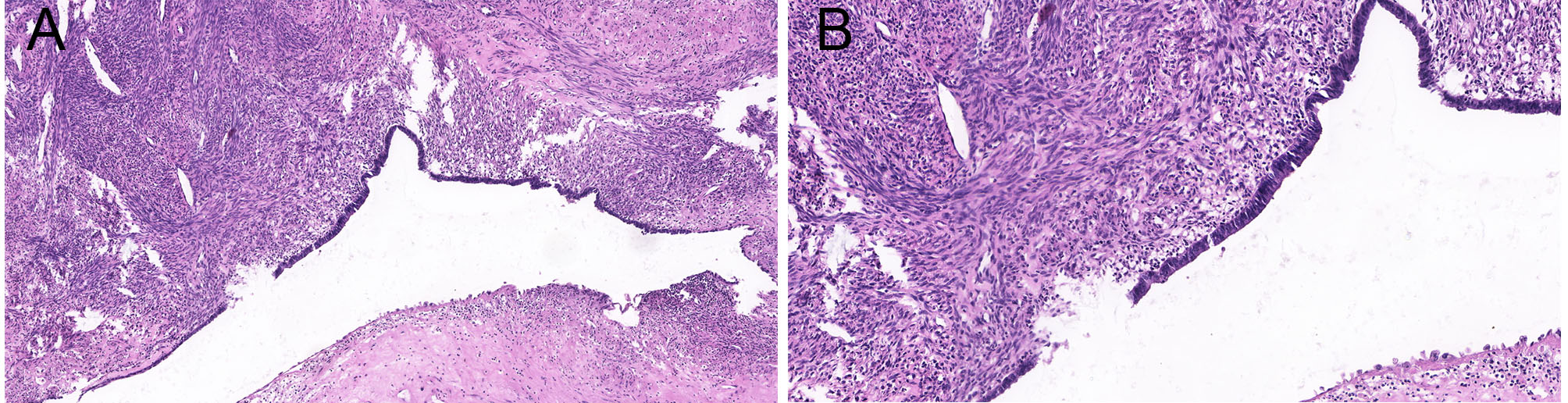 Fig. 3.
Fig. 3.
Histologic findings of cystic adenomyosis: hematoxylin and eosin
staining: the capsule of cystic adenomyosis is arranged by endometrial glands and
the stroma, and around the capsule, the organization has muscular wrapping
(A: 50
CA can be divided into juvenile CA (JCA) and adult CA. JCA is a disease that
causes severe dysmenorrhea in women less than 30 years of age with no history of
uterine surgery, and imaging findings show cystic lesions with a diameter of
Injury to the junctional zone (JZ) caused by uterine surgery may be an important factor in the occurrence of adenomyosis [11], resulting in the invasion of the basal endometrium into the myometrium, causing regeneration, healing and changes in the size [12]. We report a rare case of CA in a patient under the age of 30 who had a history of uterine surgery and could not be diagnosed with either JCA or adult CA. She should be considered to have secondary CA associated with surgery. Our patient’s secondary adenomyoma may have been associated with JZ injury during myomectomy, leading to endometrial invasion of the myometrium. This hypothesis is supported by the fact that patients recurred only 6 months after surgery. The second surgery probably resulted in the development of CA. Adenomyoma of the uterus partly protruded from the uterine cavity and partly extended to the muscularis vessels, increasing the possibility of intraoperative JZ injury. Intraoperative ultrasound scans revealed residual tissue from secondary surgery, although intraoperative tight electrocoagulation and postoperative hysteroscopy showed no residuals. The location of the cystic lesion was the same as before, and symptoms of increased menstrual volume appeared. This confirms the possibility of surgical residual tissue or JZ injury.
In the pathology of CA, the cyst wall is composed of endometrial glands and stroma, and the surrounding wall is covered with proliferative fibroid tissue [13]. Most cases of CA are confirmed by postoperative pathology [12]. Our patient was misdiagnosed with uterine fibroids before surgery and diagnosed with CA after surgery. Similar to adenomyosis, CA has nonspecific clinical manifestations, such as dysmenorrhea, chronic pelvic pain, abnormal uterine bleeding, etc. [10]. According to a review, patients under 30 years of age with CA are characterized by drug-refractory dysmenorrhea, whereas those over 30 years of age mainly suffer from chronic pelvic pain or sometimes abnormal uterine bleeding [14]. Dadhwal et al. [15] reported two cases of dysmenorrhea in patients under 30 years of age who had no changes in menstrual volume. Pontrelli et al. [16] reported a 27-year-old patient with CA who had increased menstrual volume with dysmenorrhea. We reported for the first time a case of CA in a patient under 30 years of age, whose main clinical manifestation was increased menstrual volume without dysmenorrhea.
Ultrasound is the most commonly used imaging method in the diagnosis of adenomyosis, and it can distinguish between adenomyomas and myomas. The accuracy of ultrasound depends on operator experience and expertise and the quality of the equipment [17]. The sensitivity of transvaginal or transanal ultrasound is higher than that of transabdominal ultrasound [18]. Koukoura et al. [19] reported a case in which transabdominal ultrasound misdiagnosed a patient with an ovarian cyst, but transanal ultrasound and magnetic resonance imaging (MRI) correctly diagnosed CA. MRI can confirm a diagnosis made by ultrasound [20]. MRI can accurately assess the contents of CA, endometrial and muscular tissue, and the junction area. The fluid in the cysts shows a high signal intensity on T1-weighted images, and the surrounding muscular layer shows low signal intensity on T2-weighted images [10, 21]. Hysteroscopy can diagnose adenomyosis according to the special microscopic manifestations [22]. Hysteroscopy can differentiate JCA from congenital developmental abnormalities, such as biangular uterus or ovarian cysts, and the lesions can be sampled to obtain pathological specimens for a definitive diagnosis [16]. Alabiso et al. proposed a new decision algorithm based on a 28-year-old infertility patient with uterine adenomyosis. First, the diagnosis was made by ultrasound and then confirmed by MRI. Then, hysteroscopy or laparoscopy was performed, and finally, a detailed diagnosis and treatment plan was made [12]. Unfortunately, our patient was misdiagnosed with uterine fibroids by ultrasound, and no further MRI examination was performed, nor was the mass sampled for pathological examination during hysteroscopy. MRI is very important for adenomyosis patients, especially for the identification of recurrent myoma lesions in young patients. Hysteroscopy is also very important for the diagnosis and treatment of intrauterine masses.
Surgery is an important means to treat adenomyosis, drugs can also treat it [23]. Drug treatments include GnRH-a, nonsteroidal anti-inflammatory drugs, LNG-IUS, etc. Drugs can only relieve symptoms, and recurrence can occur after drug withdrawal [24]. There are very few reports about CA drug therapy. Zhou et al. [25] reported a case of adult CA patient with significantly reduced symptoms after LNG-IUS treatment. Intraoperative residual lesions or JZ damage may cause recurrence. Surgery may lead to an adverse pregnancy history [26]. According to previous reports, conservative surgery combined with GnRH-a or an LNG-IUS can prevent recurrence and relieve dysmenorrhea and other symptoms and improve the fertility of patients [27, 28, 29]. There is a lack of reliable reports on pregnancy outcomes of CA.
In our patient, her lesion was completely excised by ultrasonic monitoring, and the mass was put into a sample bag and removed from the puncture hole by stages, further reducing residual tissue in the abdominal cavity. GnRH-a and an LNG-IUS were used for postoperative treatment to reduce symptoms and prevent recurrence. We report for the first time the successful treatment of a secondary CA patient under 30 years of age with surgery and medication. This case also has the longest follow-up to date. She was followed up for nearly 2 years without recurrence or obvious discomfort. Her pregnancy outcomes require further follow-up.
Younger patients with a history of uterine surgery may have secondary CA, and the clinical manifestations may not include the typical unbearable dysmenorrhea. For patients with unclear ultrasound diagnoses, MRI examination is very important.
For patients with intrauterine lesions, hysteroscopy can obtain a sample for pathologic diagnosis or treatment. Complete intraoperative excision of the lesion and reduction in residual tissue, combined with postoperative treatment with GnRH-a and an LNG-IUS, can effectively prevent recurrence and improve prognosis. The prognosis and pregnancy outcomes of CA are still not clear, and further study is needed.
CA, Cystic adenomyosis; GnRH-a, gonadotropin-releasing hormone analog; JCA, juvenile adult cystic adenomyosis; JZ, junctional zone; LNG-IUD, levonorgestrel-releasing intrauterine device; LNG-IUS, levonorgestrel-releasing intrauterine system; MRI, magnetic resonance imaging.
HXL, KJS, NNX and QY participated in the diagnosis and management of this case. XYJ and LS followed up the patient HXL wrote the manuscript. QY revised the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution, and written consent was obtained. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Affiliated Hospital of Qingdao University (approval number: QYFY WZLL 25965).
Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
