†These authors contributed equally.
Academic Editor: Antonio Simone Laganà
Background: To investigate whether hysteroscopic endometrial mechanical
stimulation improves pregnancy outcomes in patients undergoing in vitro
fertilization (IVF)/intracytoplasmic sperm injection (ICSI). Methods: We
conducted a systematic search in electronic databases including PubMed, Embase,
Cochrane Library, Web of Science from their inception to Feb 20th, 2021, as well
as a manual search. All publications on the impact of hysteroscopic endometrial
mechanical stimulation on IVF/ICSI outcomes were retrieved. Two reviewers
independently screened the retrieved studies using stringent inclusion and
exclusion criteria; data were subsequently extracted, and risk of bias was
assessed. Meta-analysis of the selected studies was performed using Revman 5.3.
Results: Eight studies involving 1494 patients were eligible for
inclusion, including 5 randomized controlled trials and 3 prospective
non-randomized simultaneous controlled experimental studies. We found that
compared with the control group, hysteroscopic endometrial mechanical stimulation
effectively increased live birth rate [risk ratio (RR) = 2.15, 95% confidence
interval (CI) (1.78, 2.60), p
As the prevalence of infertility increases year by year, the number of patients seeking assisted reproduction is also increasing. Despite advances in assisted reproductive technology (ART), the clinical pregnancy rate is only about 30% [1], and thus, it is imperative to improve the success rate of ART. Endometrial receptivity is a key factor influencing embryo implantation. Good blastocyst development and simultaneous development of endometrial receptivity are essential for implantation 6–10 days after ovulation, i.e., at the mid-secretory phase of menstrual cycle. Although in most cases this occurs naturally, desynchrony between blastocyst and endometrial development is noted in some women, thus affecting embryo implantation [2]. Embryo implantation is an inflammatory process; in 1907, Loeb first reported that scratching the guinea pig uterus during the pre-pregnancy phase of estrous cycle resulted in rapid growth of decidual cells, and subsequent studies proposed that the decidual growth might be attributed to the inflammatory response triggered by local injury to the endometrium [3]. In 2003, Barash et al. [4] proposed that endometrial mechanical stimulation by endometrial biopsy could improve endometrial receptivity and thus facilitate embryo implantation. The underlying mechanism is poorly understood, which may involve endometrial decidualization caused by histamine release in response to local damage to the endothelium; a large number of cytokines and growth factors involved in the process of embryo implantation are secreted during the repair of endometrial lesions [4], and local injury increases endometrial receptivity by regulating the expression of multiple genes, which may contribute to the success of embryo implantation [5].
Over the past 20 years, many studies have been conducted on endometrial mechanical stimulation by means of pipelle biopsy tube, small curette, hysteroscopy, or hysteroscopy combined with surgical instrumentation [6]. Endometrial mechanical stimulation is simple and can be performed by many gynecologists, but the procedure may cause pain and carries the risk of higher cost, endometrial injury and etc. [7]. Also, mixed results have been reported on its effect on pregnancy outcomes after embryo transfer. Some studies observed that endometrial mechanical stimulation might improve the implantation rate and live birth rate and reduce the miscarriage rate [8], while others did not found any benefits for embryo implantation [9]. Hysteroscopy provides a direct visualization of the uterine cavity and is the gold standard for the diagnosis of uterine diseases, which avoids the disadvantages of other mechanical stimulation methods that cannot detect uterine lesions. With advances in hysteroscopy techniques, hysteroscopic procedures can be performed in an outpatient setting without any anesthesia. It is of clinical relevance to determine whether it is necessary to administer endometrial mechanical stimulation that may increase the risks of pain, higher cost, and injury in patients with normal hysteroscopic findings [7], and whether this procedure can increase clinical pregnancy rate. Although several meta-analyses have been published, there is a lack of study assessing the effect of endometrial mechanical stimulation on embryo implantation in patients with normal hysteroscopic findings. Thus, this meta-analysis was performed to provide a more scientific basis for clinical decision-making.
(1) study type: randomized controlled trials (RCTs) and non-RCTs; (2) study subjects: infertile patients undergoing in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI), regardless of nationality, ethnicity, or duration of illness; no acute inflammation or systemic disease; (3) interventions: hysteroscopy combined with endometrial mechanical stimulation followed by IVF/ICSI in the treatment group; while hysteroscopy or other examination excluded endometrial lesions and IVF/ICSI subsequently in the control group; (4) outcome measures: live birth rate, clinical pregnancy rate, and abortion rate; (5) published studies or with publication year; with adequate description of sample size and results.
(1) retrospective studies; (2) studies with no control group, or with significant baseline differences between groups; (3) duplicate publications, studies with flawed design, incomplete data, or were of low-quality; (4) studies with insufficient descriptions despite contact with the author; (5) studies with incorrect and uncorrectable statistical methods or unusable data; (6) animal experiments, case reports or literature reviews.
The following electronic databases were searched from their inception to Feb 20th, 2021: PubMed, Embase, Cochrane Library, and Web of Science; and manual search was also performed. The search terms were ((“Hysteroscopy”[Mesh]) OR (Hysteroscop*) OR (Endoscop*) OR (mini-hysteroscop*) OR (minihysteroscop*)) AND ( (endometrial biopsy) OR (endometrial injury) OR (endometrial trauma)) OR (mock embryo transfer) OR (endometrial sampling) OR (endometrial local injury) OR (endometrial priming) OR (endometrial harm) OR (endometrial wound) OR (endometrial lesion) OR (endometrial damage)) AND ((assisted reproducti*) OR (IVF) OR (in vitro fertili*) OR (in vitro fertil*) OR (ICSI) OR (intracytoplasmic sperm injection) OR (embryo* AND transfer*) OR (blastocyst* AND transfer*) OR (FET) OR (“Reproductive Techniques, Assisted”[Mesh])). A total of 912 studies published in English were retrieved.
Study selection and data extraction was conducted by two reviewers independently. First, they screened the title and abstract for potentially relevant studies. Next, they read through the entire study to evaluate its eligibility for inclusion. Then, resultant studies from the two reviewers were compared; any discrepancies between the two reviewers were resolved through discussion with a third reviewer, who was an expert in the field. Data were extracted from each study according to the data extraction table. If there was any missing data, the corresponding author was contacted to obtain the required information. If a study group had multiple articles based on similar patients and using the same measures, only the largest or most recent article was included. The data extracted comprised the following: (1) basic information such as name of first author and year of publication; (2) study design, and parameters for risk of bias assessment; (3) basic patient characteristics, such as sample size (treatment/control group), patient age, and nationality; (4) details of intervention strategies, including timing of hysteroscopy and specific methods of endometrial mechanical stimulation; (5) outcome data including live birth rate (i.e., the ratio between the number of live births beyond 22 weeks of gestation and the total number of transfers); clinical pregnancy rate (clinical pregnancy defined as the ultrasound presence of a fetal heartbeat in the gestational sac); and abortion rate (i.e., the ratio between the number of early pregnancy losses and the total number of clinical pregnancies).
We used Review Manager 5.3 (The Cochrane Collaboration, Oxford, UK) [12] to
perform meta-analysis. Continuous variables were presented as mean differences
(MD), while categorical variables were reported as relative risks (RR). For each
outcome measure, point estimates and 95% confidence intervals (95% CI) were
also calculated. Heterogeneity across studies was evaluated using the I
A total of 912 relevant studies were initially retrieved. After careful screening, 8 studies [6, 8, 13, 14, 15, 16, 17, 18] met the eligibility criteria including 5 RCTs [8, 13, 14, 16, 18] and 3 prospective non-randomized simultaneous controlled experimental studies [6, 15, 17]. Totally 1494 patients were included for analysis. The search process and the number of studies obtained in each step were presented in a flow chart in Fig. 1.
 Fig. 1.
Fig. 1.Flow chart of study selection.
Tables 1,2 (Ref. [6, 8, 13, 14, 15, 16, 17, 18]) presented the basic characteristics of the included studies. A total of 1494 patients from 8 countries or regions were included; of the 8 articles, 2 [17, 18] had impact factors greater than 3 points, while the quality of the other articles was relatively low. Fig. 2 presented the assessment of risk of bias for the included RCTs. All RCTs described the process of random sequence generation, and all were of low risk; 3 RCTs [8, 13, 14] used sealed envelopes for allocation concealment and were rated as low risk, while the other 2 RCTs [16, 18] did not mention allocation concealment and were rated as uncertain risk; only 1 RCT [13] achieved double-blindness and was rated as low risk, while the other RCTs were rated as high risk because blinding was not feasible; no loss to follow-up was reported in 3 RCTs [8, 13, 14], and the loss to follow-up rate was lower than 20% in the other 2 RCTs [16, 18], so all RCTs were rated as low risk in terms of data integrity; the study protocol was not available in all RCTs, and thus the risk of selective reporting was considered as uncertain; all RCTs appeared to be free of other sources of bias, and were regarded as low risk. The methodological quality assessment of non-RCTs was presented in Table 3 (Ref. [6, 15, 17]). All non-RCTs scored at least 12, and therefore all could be included in our meta-analysis.
| Study | Study design | Study quality | Country | No. of patients (T/C) | Mean age | Interventions | Outcome measures | |
| (T/C, years) | T | C | ||||||
| Berntsen, S 2020 [16] | RCT | IF 1.868 | Denmark | 95/95 | 34.0 |
hysteroscopy + endometrial injury in the follicular phase of the cycle before ovulation induction | none | ②③④ |
| Timur Gürgan 2019 [18] | RCT | IF 3.218 | Turkey | 124/115 | 34.31 |
hysteroscopy + endometrial injury in the follicular phase of the cycle before ovulation induction | none | ①②③④⑤⑥ |
| Dejan Mitić 2018 [6]a | Prospective | MIN 22 | Serbia | 40/151 | 32.78 |
hysteroscopy + endometrial injury in the follicular phase before ovulation induction | none | ①②③⑥ |
| Dejan Mitić 2018 [6]b | Prospective | MIN 22 | Serbia | 40/41 | 32.78 |
hysteroscopy + endometrial injury in the follicular phase before ovulation induction | hysteroscopy alone | ①②③⑥ |
| Charalampos Siristatidis 2017 [15] | Prospective | MIN 22 | Greece | 51/52 | 36 (27–42)/36 (28–41) | hysteroscopy + endometrial injury in the follicular phase of the cycle before implantation | none | ②③④⑤ |
| Ahmad Mahran 2016 [8] | RCT | IF 0 | Egypt | 200/200 | 31.4 |
hysteroscopy + endometrial injury in the follicular phase of the cycle before implantation | hysteroscopy alone | ①③④⑤⑥ |
| Amal Shohayeb 2012 [13] | RCT | IF 1.868 | Egypt/Saudi Arabia | 100/100 | 30.7 |
hysteroscopy + endometrial injury in the follicular phase of the cycle before implantation | hysteroscopy alone | ①③④⑤ |
| Narvekar, Sachin A 2010 [14] | RCT | IF 0 | India | 49/51 | 32.16 |
follicular-phase hysteroscopy + intraoperative endometrial injury + luteal-phase endometrial injury before implantation | hysteroscopy alone | ①③④ |
| Huang, S. Y. 2011 [17] | Prospective | IF 3.235 | Taiwan | 6/24 | 34 |
hysteroscopy + endometrial injury in the follicular phase before ovulation induction | none | ①③⑤ |
| Notes:
RCT, randomized controlled trial; prospective, prospective non-randomized
simultaneous controlled experimental study; IF, impact factor; MIN,
methodological index for non-randomized studies; T, treatment group; C, control
group; a, in the study of Dejan Mitić 2018, the intervention group included
40 patients who received LEI (local endometrial injury) during hysteroscopy, and
the control group included 151 patients who did not receive hysteroscopy or LEI;
b, in the study of Dejan Mitić 2018, the intervention group included 40
patients who received LEI during hysteroscopy, and the control group included 41
patients who received hysteroscopy without LEI. ①embryo implantation; ②biochemical pregnancy; ③clinical pregnancy; ④live birth; ⑤abortion; ⑥multiple pregnancy. | ||||||||
| Study | Specific methods | Participants | Embryo transfer |
| Berntsen, S 2020 [16] | Hysteroscopic biopsy forceps stimulate posterior uterine wall. | FET | |
| Timur Gürgan 2019 [18] | First on the fundus by cutting transversally into the endometrium. Later, three or four vertical incisions were performed 0.5 cm apart, on the anterior and posterior walls of the uterus, 1–1.5 cm away from the fundus and with one cut for each lateral wall by scissors. | FET or TET according to the patient’s indications | |
| Dejan Mitić 2018 [6] | 10–15 mm in length throughout the whole endometrial thickness in a transversal direction with a springle bipolar electrode. | First/second IVF | FET |
| Charalampos Siristatidis 2017 [15] | Three cuttings of 0.5 cm on the front endometrial wall, 1 cm lower of the endometrial fundus level. | FET | |
| Ahmad Mahran 2016 [8] | Pipelle endosampler catheter scratching of fundus and posterior wall of uterine cavity was done three times. | first IVF/ICSI | FET |
| Amal Shohayeb | Curettage of the fundus and posterior wall once by Novak curette. | FET | |
| 2012 [13] | |||
| Narvekar, Sachin A 2010 [14] | Pipelle endosampler catheter was rotated 360 degrees and moved up and down four times after withdrawing the piston. | FET | |
| Huang, S. Y. 2011 [17] | A local injury on the posterior endometrium at mid-line 10–15 mm from the fundus by a claw forceps, the depth and width of the injured site was 2 × 2 mm. | FET | |
| FET, fresh embryo transfer; TET, thawing embryo transfer; IVF, in vitro fertilization; ICSI, intracytoplasmic sperm injection. | |||
| Non-comparative study | |||||||||
| Study | A clearly stated aim | Inclusion of consecutive patients | Prospective collection of data | Endpoints appropriate to the aim of the study | Unbiased assessment of the study endpoint | Follow-up period appropriate to the aim of the study | Loss to follow-up less than 5% | Prospective calculation of the study size | Score |
| Dejan Mitić 2018 [6]a | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | / |
| Dejan Mitić 2018 [6]b | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | / |
| Charalampos Siristatidis 2017 [15] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | / |
| Huang, S. Y. 2011 [17] | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 0 | / |
| Additional criteria in the case of comparative study | |||||||||
| Study | An adequate control group | Contemporary groups | Baseline equivalence of groups | Adequate statistical analyses | Score | ||||
| Dejan Mitić 2018 [6]a | 2 | 2 | 2 | 2 | 22 | ||||
| Dejan Mitić 2018 [6]b | 2 | 2 | 2 | 2 | 22 | ||||
| Charalampos Siristatidis 2017 [15] | 2 | 2 | 2 | 2 | 22 | ||||
| Huang, S. Y. 2011 [17] | 2 | 2 | 2 | 2 | 21 | ||||
| Notes: a, in the study of Dejan Mitić 2018, the intervention group included 40 patients who received LEI (local endometrial injury) during hysteroscopy, and the control group included 151 patients who did not receive hysteroscopy or LEI; b, in the study of Dejan Mitić 2018, the intervention group included 40 patients who received LEI during hysteroscopy, and the control group included 41 patients who received hysteroscopy without LEI. | |||||||||
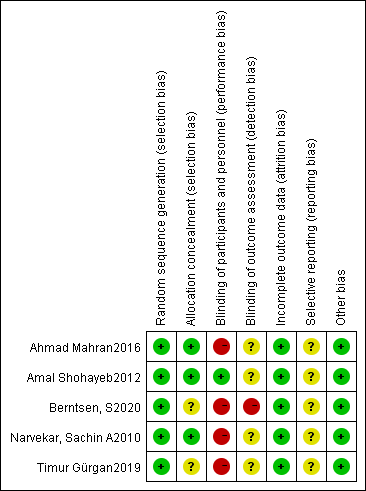 Fig. 2.
Fig. 2.Assessment of risk of bias for the included RCTs.
Six studies were included for this analysis [8, 13, 14, 15, 16, 18]. Due to sufficient
homogeneity across studies [p = 0.81, I
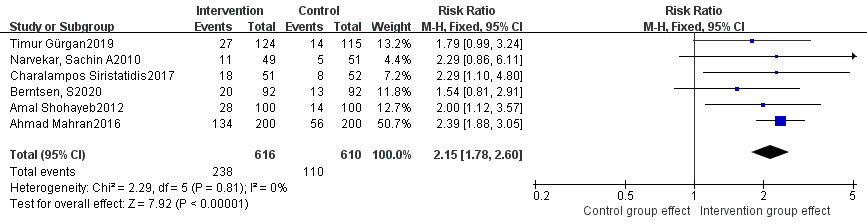 Fig. 3.
Fig. 3.Comparison of live birth rate between intervention group and control group.
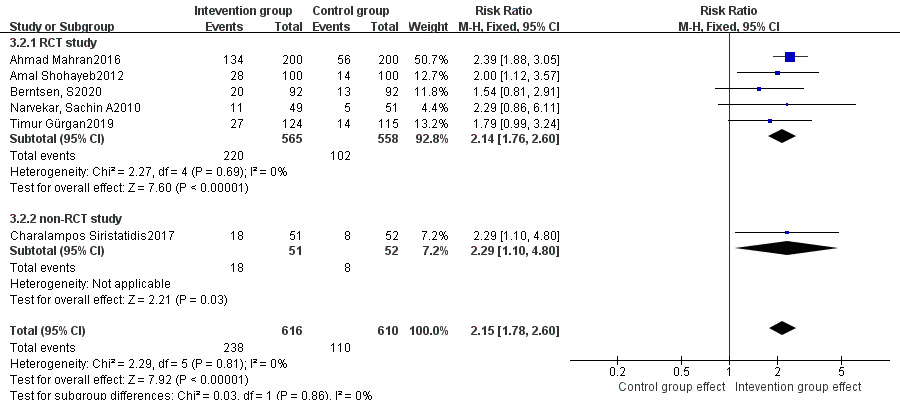 Fig. 4.
Fig. 4.Subgroup analysis of live birth rate between intervention group and control group stratified by RCT vs. non-RCT.
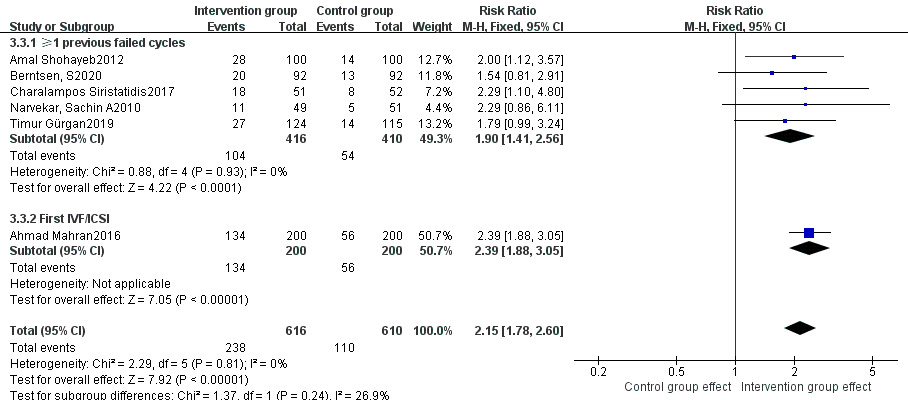 Fig. 5.
Fig. 5.Subgroup analysis of live birth rate between intervention group and control group stratified by prior history of failed cycles.
Eight studies were included for this analysis [6, 8, 13, 14, 15, 16, 17, 18]. Due to significant
heterogeneity across these studies [p
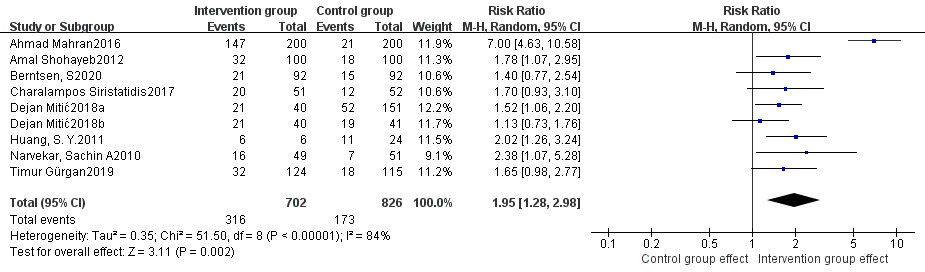 Fig. 6.
Fig. 6.Comparison of clinical pregnancy rate between intervention group and control group.
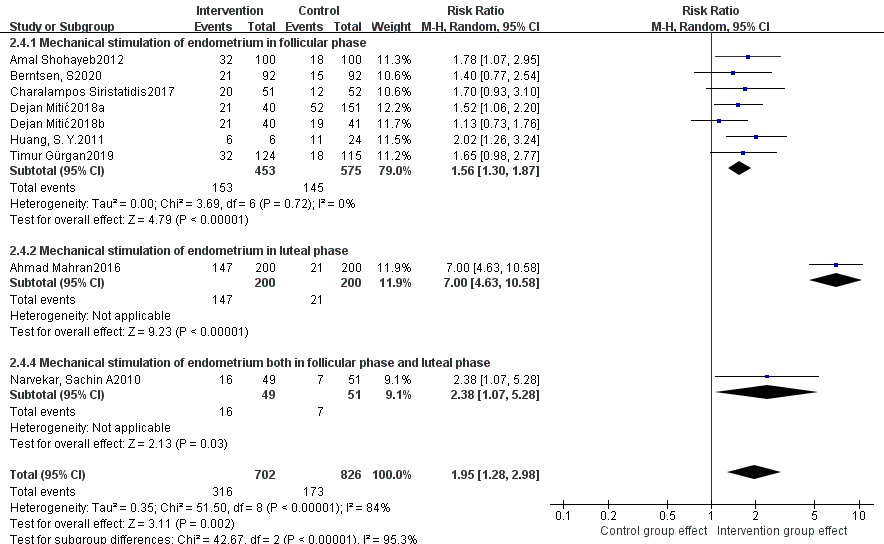 Fig. 7.
Fig. 7.Subgroup analysis of clinical pregnancy rate between intervention group and control group stratified by timing of stimulation.
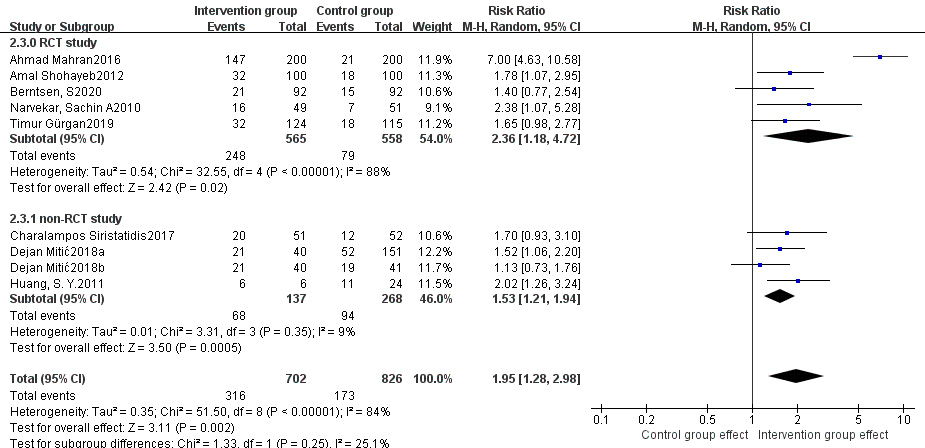 Fig. 8.
Fig. 8.Subgroup analysis of clinical pregnancy rate between intervention group and control group stratified by RCT vs non-RCT.
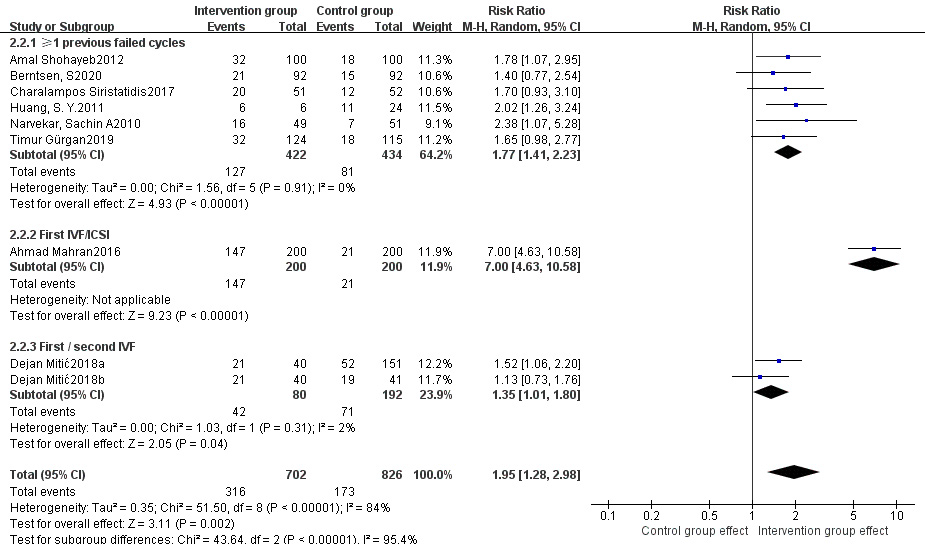 Fig. 9.
Fig. 9.Subgroup analysis of clinical pregnancy rate between intervention group and control group stratified by prior history of failed cycles.
Five studies were included for this analysis [8, 13, 15, 17, 18]. Due to
statistical homogeneity across studies (p = 0.89, I
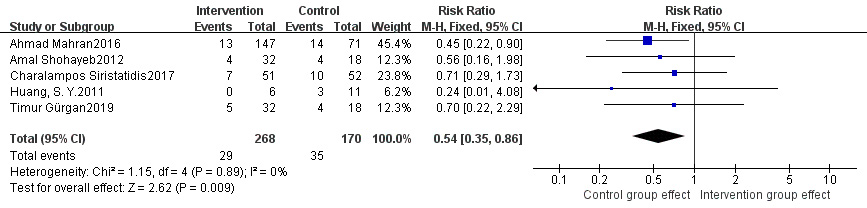 Fig. 10.
Fig. 10.Comparison of abortion rate between intervention group and control group.
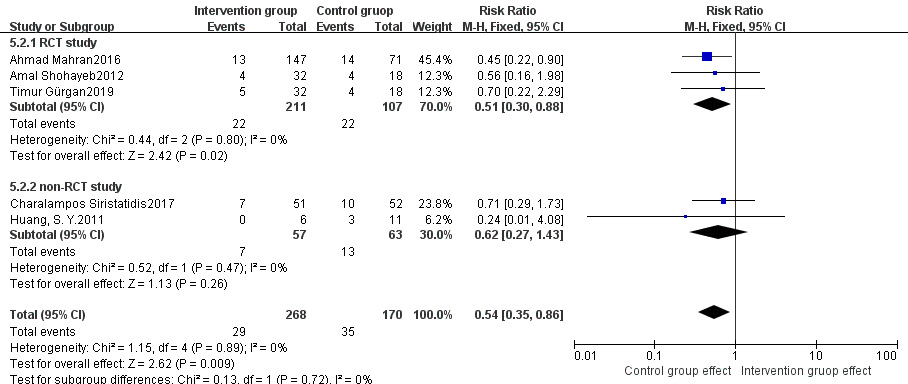 Fig. 11.
Fig. 11.Subgroup analysis of abortion rate between intervention group and control group stratified by RCT vs non-RCT.
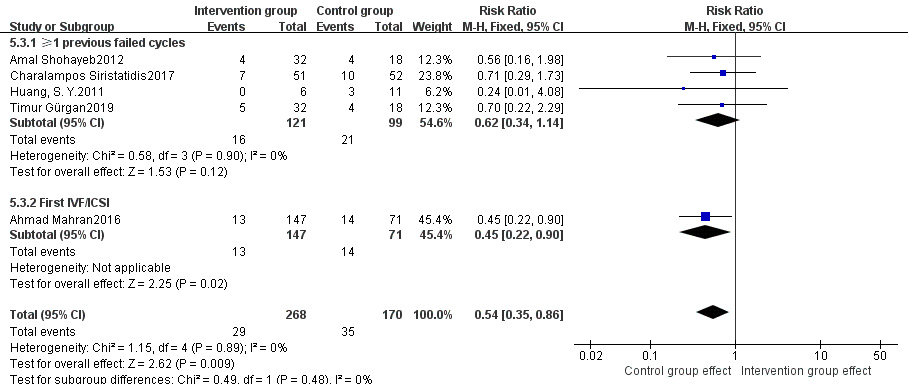 Fig. 12.
Fig. 12.Subgroup analysis of abortion rate between intervention group and control group stratified by prior history of failed cycles.
Endometrial mechanical stimulation is a procedure that is easy to perform. In 2003, Barash et al. [4] proposed that endometrial mechanical stimulation might improve endometrial receptivity and thus facilitate embryo implantation. Since then, a series of related studies have been published, in which endometrial mechanical stimulation was achieved by curette scratching, pipelle biopsy, hysteroscopy, or hysteroscopy combined with surgical instrumentation. Given its simplicity, it may become a very promising clinical approach to improve pregnancy outcomes in patients undergoing IVF/ICSI. However, its role in ART is controversial. Some believe that it should be used with caution because this is an invasive procedure and may cause pain and discomfort, higher cost and increase the risk of endothelial injury leading to Asherman syndrome [7]. But others argue that there is evidence supporting the efficacy of endometrial mechanical stimulation in improving the implantation rate and live birth rate and reducing the miscarriage rate [8]. Recent meta-analyses [19, 20] also reported mixed results on the role of endometrial mechanical stimulation. For this reason, we performed a meta-analysis to assess the impact of endometrial mechanical stimulation on IVF/ICSI outcomes in patients with normal hysteroscopic findings, so as to provide more evidence for real-world clinical practice.
In contrast to prior studies [20], only patients with normal hysteroscopic findings were included in our study, and this homogeneity of participants may provide targeted guidance for clinical management of such patients. Our analyses indicated that endometrial mechanical stimulation might improve live birth rate, clinical pregnancy rate and reduce abortion rate in patients with normal hysteroscopic findings.
According to subgroup analyses stratified by timing of stimulation, endometrial mechanical stimulation given in the follicular and/or luteal phase was associated with higher clinical pregnancy rates, and the benefit was even greater when given in the luteal phase. It has been shown that mechanical stimulation in the presence of progestins in the luteal phase may induce more effective decidualization response [21]. And endometrial mechanical stimulation on days D21-24 of menstrual cycle may trigger an inflammatory response characterized by an influx of macrophages and an increase in pro-inflammatory cytokines, which is positively correlated with pregnancy outcomes [22]. Besides, endometrial mechanical stimulation administered in the luteal phase may relieve patient discomfort [23]. Thus, endometrial mechanical stimulation in the luteal phase is a promising approach for clinical use. However, as only one study involving endometrial mechanical stimulation in the luteal phase was included in this meta-analysis, more studies are needed to clarify whether stimulation in the luteal phase is superior to that given in the follicular phase.
Moreover, subgroup analyses stratified by study type (RCT vs. non-RCT) indicated that, significantly higher live birth rate and clinical pregnancy rate were noted in the treatment group compared with those of the control group in both RCTs and non-RCTs, and a significantly lower abortion rate was also found in the treatment group in RCTs, but not in non-RCTs. Due to the low incidence of abortion and the small sample size of studies reporting abortion rate, it may be insufficient to identify statistically significant difference in abortion rate, and more relevant RCTs or non-RCTs are needed to confirm the reliability of this finding.
Besides, we also performed subgroup analyses stratified by prior history of failed cycles. Hysteroscopic endometrial mechanical stimulation significantly improved the live birth rate and clinical pregnancy rate both in patients undergoing their first IVF/ICSI cycle and in those having at least one failed cycle. The clinical pregnancy rate was even higher in patients in their first IVF/ICSI cycle. A significantly lower abortion rate was found in patients undergoing their first IVF/ICSI cycle, but not in those with at least one failed cycle. It has been reported that hysteroscopic endometrial mechanical stimulation may improve pregnancy outcomes in patients who have failed previous cycles [24]. In our study, for the first IVF/ICSI cycle, luteal-phase mechanical stimulation, which was given after endometrial lesions had been excluded by hysteroscopy, dramatically improved clinical pregnancy rate. Therefore, hysteroscopy combined with luteal-phase mechanical stimulation may be considered prior to the first IVF/ICSI cycle. However, as only one study involving luteal-phase endometrial mechanical stimulation in patients undergoing their first IVF/ICSI cycle was included in this meta-analysis, more studies are required to better define its role in clinical practice.
We acknowledge that our analysis has several limitations. Firstly, as some patients declined to receive the intervention, complete randomization was sometimes not feasible and three non-RCTs were included in our analysis, which may compromise the level of evidence. Secondly, no interventions were given in the control group of some RCTs, and thus blinding of participants was not feasible. And different stimulation methodologies were used in the included studies, such as different instruments (hysteroscopic scissors, biopsy forceps, bipolar electrodes, Novak curette or pipelle tube), sites (the posterior wall of the uterus alone; the fundus, anterior/posterior walls and both sides; or the fundus and posterior wall), as well as length, width, depth and quantity of stimulation. As these differences may induce variable endometrial responses and then have different effects on pregnancy outcomes, performance bias may exist. Most published studies were performed in patients with abnormal hysteroscopic findings and the incidence of unexpected hysteroscopic lesions was up to 45.1% [25]; the presence of hysteroscopic abnormalities may confound the baseline characteristics of participants, and such studies cannot be included in our analysis. And thus, only a small number of studies were included, which may cause a lack of effective way to guide the method for endometrial mechanical stimulation. Thirdly, fresh embryo transplantation was performed in 7 of the 8 included studies, and only in the Timur Gürgan 2019 [18] study fresh or thawed embryo transplantation was performed according to patient’s preferences. Thus, our findings have limited implications for patients undergoing frozen-thawed embryo transplantation. Finally, only one study assessing luteal-phase endometrial mechanical stimulation prior to the first IVF/ICSI cycle in patients with normal hysteroscopic findings was included in this meta-analysis, and the stability of our findings may be affected by the large sample size and high weights of this study. Thus, more high-quality studies are needed.
In conclusion, based on the available evidence, endometrial mechanical stimulation may improve live birth rate, clinical pregnancy rate and reduce abortion rate in patients with normal hysteroscopic findings who are undergoing IVF/ICSI. The benefits may be even greater if this therapy is given in the luteal phase and in patients who are in their first IVF/ICSI cycle in patients with normal hysteroscopic findings. However, due to the limited quantity and quality of the included studies, more high-quality RCTs are required to confirm these findings and further define the appropriate indications, optimal timing, instrument, site and extent of endometrial mechanical stimulation.
HC, LJL and JL designed the research study. LJL and JL performed the research and collected the data. LZX provided help and advice to LJL and JL. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
We are grateful to all those who provided useful assistance in the writing of this manuscript.
This meta-analysis was part of Study on the risk factor assessment tool for premature ovarian failure, which was funded by the Technological Innovation and Development Program of Chengdu Bureau of Science and Technology (2019-YFYF-00101-SN); and was also part of Creating a risk factor assessment tool for premature ovarian failure, which was funded by the Key Science Research Program of Health Commission of Sichuan Province (20ZD008).
The authors declare no conflict of interest.
