Background: Amniocentesis (AC) is the most commonly used
invasive prenatal test. The aim of this study was to determine which were the
most common indications for AC, chromosomal abnormalities in relation to the age
of the mother and complications as a result of AC. Methods: This is a
retrospective thirteen-year cohort study including pregnant women who underwent
AC in the period from 2008 to 2020. Patients were divided into two groups: below
and above 35 years of age of mother at the time of AC. Results: During
study period 2213 AC were performed, out of which 759 (34.29%) were in mother
below, and 1454 (65.70%) above 35 years of age (p
Amniocentesis (AC) is the aspiration of amniotic fluid from the amniotic cavity and is used for the prenatal diagnosis of aneuploidy, congenital diseases or infections. Genetic AC is performed to confirmed or exclusion of aneuploidy. AC is the most commonly used invasive prenatal test [1]. AC, in our institution, is performed between 16 and 18 weeks of gestation, up to 20 weeks at the latest. During this period, the highest percentage of successful cultures was obtained from amniotic fluid cells, and the procedure itself was facilitated due to the size of the uterus [2]. The analysis is performed on fetal cells from the obtained amniotic fluid and chromosome abnormalities are detected [3].
The indicators for AC are: increased risk of fetal aneuploidy (combined test,
non-invasive prenatal test, abnormalities on ultrasound examination), previous
fetus with abnormalities, existence of chromosome translocations in the family
history, increased risk for genetic diseases (autosomal recessive or autosomal
X-related diseases) one or both parents, TORCH infection [4], age of the mother
(
AC is a relatively safe procedure with minimal risk to the patient. Chorioamnionitis occurs in less than 0.1% of cases. In some cases, transient vaginal bleeding may occur, as well as amniotic fluid leakage, while the risk of miscarriage as the most severe complication is about 1% [4, 7].
The implementation of effective screening tests decreased the need for AC, thus, reducing the risk of procedure-related complications. Screening tests, however, have limitations which include both false positive and false negative results. While more advanced procedures such as cell-free fetal DNA testing from maternal blood are available, AC are still being used extensively for prenatal diagnosis in most countries. It seems that the numbers of AC have been increasing because of delayed childbearing age, but in fact the use of noninvasive prenatal testing has actually led to a reduction in the number of invasive testing [8, 9, 10, 11, 12].
The aim of this study was to determine the most common indications for AC, chromosomal abnormalities in relation to maternal age, and complications as a result of AC.
This retrospective cohort study included pregnant women who undergone of AC during the period between 1 January 2008 and 31 December 2020 at the Department of Maternal Fetal Medicine, Clinic for Gynaecology and Obstetrics, University Clinical Centre Tuzla.
The survey was approved by the Ethics Committee of the University Clinical Centre Tuzla. AC was performed with written informed consent.
Inclusion criteria were pregnant women who underwent of AC between 16 to 20 weeks of gestation.
Exclusion criteria were unsuccessful amniocentesis, no amniotic fluid is obtained after two consecutive attempts as well as a small amount of amniotic fluid is obtained, blood-stained amniotic fluid is obtained, amniocentesis after 20 weeks of gestation as well as late amniocentesis.
One hundred and forty-two patients (6.03%) were excluded from study, because above mentioned criteria. Patients were divided into two groups: below and above 35 years of age of mother at the time of the AC.
All Data on indications, outcome and complications of AC were collected from medical records (protocol of performed amniocentesis).
Obstetrical data included maternal age at time of AC, gestational age and indications for AC (advanced maternal age of 35 years or older, abnormal ultrasound findings (nuchal translucency, choroid plexus cyst, pyelctasia), other ultrasound markers, fetal anomalies, positive screening for maternal serum biochemical markers, familial chromosomal diseases, previous pregnancy with fetal anomalies, previous pregnancy with chromosomal abnormalities). There was more than one indication for AC in many cases.
The AC Outcome included fetal gender and frequency and type of chromosomal abnormalities (Down, Edwards, Patau and Turner syndrome, sex chromosome abnormalities, triploidy, monosomy, mosaics, translocations and inversion of chromosome 9 and others).
The short-term AC-related complications we included in the study were: frequency of spontaneous abortion, missed abortion, and premature rupture of the membrane. Short-term complications that occurred in the period of four weeks after the intervention were taken into account.
Prior to AC, each patient underwent ultrasound examination to determine placental location, fetal vitality, confirm gestational age, perform complete fetal biometrics, and locate the optimal puncture site.
The AC process involved two obstetricians, with adequate expertise and experience, at Department of Maternal Fetal Medicine (GG and LA).
After adequate preparation of the mother’s abdomen with an antiseptic solution (iodine povidone, further intervention was performed under the ultrasound guidance using the free-hand technique [8]. Needles are most commonly used from 20 to 22 gauges [9].
The largest pocket of amniotic fluid without the umbilical cord and fetal parts was found and an amniotic puncture was performed.
About 20 mL of amniotic fluid was aspirated into a sterile syringe and the first 1 to 2 mL of amniotic fluid was removed due to possible contamination by maternal cells.
The obtained amniotic fluid was sent to the Cytogenetic Laboratory for cytogenetic analysis.
After the intervention, the fetal heart rate was checked. In case of Rh isoimmunization, the mother receives Immunorho® (Kedrion, Antimo (Napoli) Italy), anti-D (Rho) immune globulin (human) within 72 hours after the intervention. The patient was observed for two hours after amniocentesis, and then discharged in the absence of immediate complications, asked to inform the department if any complications occurred after the procedure for four weeks.
Descriptive statistics (mean value, standard deviation (SD) and percentage) were used in statistical data processing. Descriptive statistics are used to describe the basic features of the data in our study. The statistical significance of the difference between mean values of variables in the groups was tested by ANOVA test.
The Student’s t-test (statistical comparison test) and the z-test
(proportional test) were used to determine statistical significance of the
difference between a set of two data (indications for amniocentesis and frequency
and type of chromosomal abnormalities between two groups of patients).
Statistically significant difference was set at p
During the thirteen-year period, 2213 ACs were performed, out of which 759
(34.29%) were in the mother below 35 years of age, and 1454 (65.70%) above 35
years of age (p
One hundred and forty-two patients (6.03%) were excluded from study. During the
observed period, significant changes were not observed in the prevalence of
excluding patients from the study (p
During the observed period, significant changes in the prevalence of AC were
observed (p
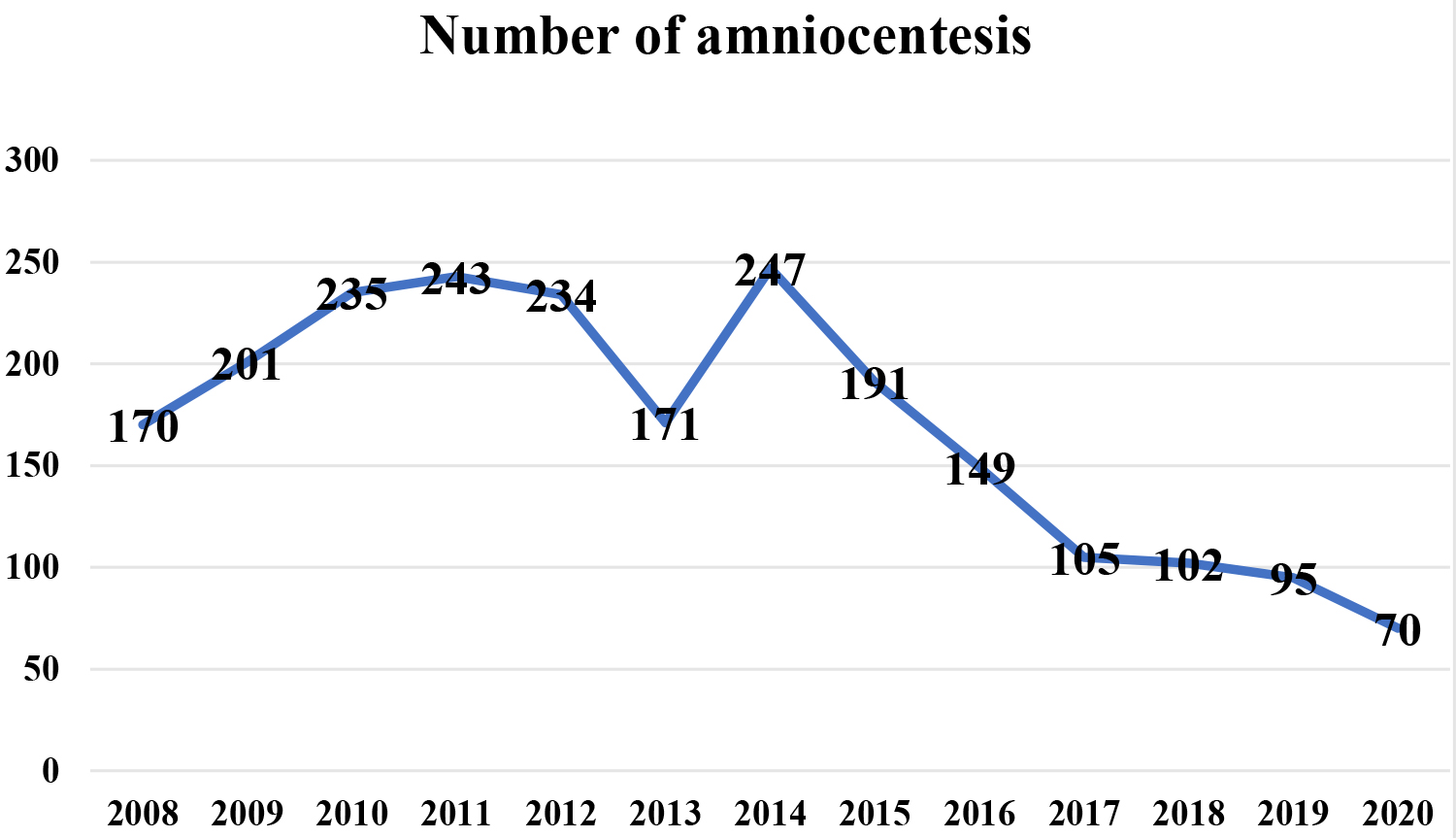 Fig. 1.
Fig. 1.Prevalence of amniocentesis during observed period.
Indications for AC by maternal age are shown in Table 1. The most common indication for AC in the group above 35 years was advanced maternal age (93.9%). The most common indication in the group below 35 years was familial chromosomal diseases (29.91%) (Table 1).
| Indications for amniocentesis | Total | p | |||||
| N (%) | N (%) | N (%) | (Two Proportions z-test) | ||||
| Nuchal translucency | 147 | 19.37 | 17 | 1.17 | 164 | 7.09 | |
| Chorioid plexus cyst | 74 | 9.75 | 4 | 0.28 | 78 | 3.37 | |
| Pyelectasis | 40 | 5.27 | 4 | 0.28 | 44 | 1.9 | |
| Other ultrasound abnormalities | 72 | 9.48 | 8 | 0.55 | 80 | 3.45 | |
| Maternal serum markers | 79 | 10.41 | 17 | 1.17 | 96 | 4.15 | |
| Familial chromosomal diseases | 227 | 29.91 | 29 | 1.99 | 256 | 11.07 | |
| Previous pregnancy with fetal anomalies | 73 | 9.62 | 7 | 0.48 | 80 | 3.46 | |
| Previous pregnancy with chromosomal abnormalities | 41 | 5.40 | 7 | 0.48 | 48 | 2.07 | |
| Other* | 10 | 1.32 | 1 | 0.07 | 11 | 0.47 | |
| Total indications | 763 | 33.01 | 1548 | 66.98 | 2311 | 100 | |
| *X-rays in pregnancy, teratogenic drugs in pregnancy, Robertson translocation in husband, cytomegalovirus and toxoplasma infection in pregnancy. | |||||||
Indications for the entire sample were advanced maternal age in 1454 cases (62.91%), nuchal translucency in 164 cases (7.09%), chorioid plexus cyst in 78 cases (3.37%), pyelectasis in 44 cases (1.9%), other ultrasound abnormalities (cystic hygroma, ventriculomegaly, single umbilical artery, reverse flow in ductus venosus, hyperechogenic bowel, omphalocele, intrauterine growth restriction, short femur, echogenic intracardiac focus) in 48 cases (2.07%), fetal anomalies in 32 cases (1.38%), positive maternal serum markers in 96 cases (4.15%), familial chromosomal diseases in 256 cases (11.07%), previous pregnancy with fetal anomalies in 80 cases (3.46%), previous pregnancy with chromosomal abnormalities in 48 cases (2.07%) and other (exposure to X-rays in pregnancy, teratogenic drugs in pregnancy, Robertson translocation in husband, cytomegalovirus and toxoplasma infection in pregnancy in 11 cases (0.47%) (Table 1).
In all indications there is a statistically significant difference between the
two age groups of pregnant women, all these indications are more prevalent in the
group of pregnant women younger than 35 years (p
Outcome of AC included fetal gender, frequency and type of chromosomal abnormalities according to mother’s age are shown in Table 2.
| Type of abnormalities | Total | p | |||||
| N (%) | N (%) | N (%) | (Two Proportions z-test) | ||||
| Numerical | 23 | 3.03 | 26 | 1.78 | 49 | 48.03 | 0.058 |
| Down syndrome | 11 | 1.45 | 16 | 1.10 | 27 | 26.47 | 0.477 |
| Edwards syndrome | 4 | 0.53 | 3 | 0.21 | 7 | 6.86 | 0.206 |
| Patau syndrome | 1 | 0.132 | 1 | 0.069 | 2 | 1.96 | 0.638 |
| Turner syndrome | 3 | 0.40 | 1 | 0.07 | 4 | 3.92 | 0.085 |
| Other sex chromosome abnormalities* | 0 | 0 | 3 | 0.2 | 3 | 2.94 | 0.211 |
| Triploidy | 1 | 0.132 | 0 | 0 | 1 | 0.98 | 0.167 |
| Monosomy** | 2 | 0.26 | 0 | 0 | 2 | 1.96 | 0.05 |
| Mosaics | 1 | 0.132 | 2 | 0.138 | 3 | 2.94 | 0.968 |
| Structural | 14 | 1.84 | 39 | 2.68 | 53 | 51.96 | 0.222 |
| Translocations*** | 1 | 0.132 | 1 | 0.07 | 2 | 1.96 | 0.638 |
| Other inversions**** | 0 | 0 | 2 | 0.138 | 2 | 1.96 | 0.307 |
| Chromosome 9 inversion | 11 | 1.45 | 28 | 1.93 | 39 | 38.23 | 0.416 |
| Other structural abnormalities of chromosomes***** | 2 | 0.26 | 8 | 0.55 | 10 | 9.8 | 0.342 |
| Total | 37 | 5.01 | 65 | 4.47 | 102 | 4.6 | 0.567 |
| *47, XXX (2), 47, XXY (1), **45,XX (1), 45 XY (1), ***t (13;19), t (3;12), ****Chromosomes 4 (1), 12 (1), *****Chromosomes 1 (1), 5 (1), 6 (1), 9 (1), 12 (1), 13 (1), 16 (1), 21 (3). | |||||||
The male to female ratio was 1124 (52.30%):1025 (47.69%).
The normal karyotype was present in 2111 (95.39%) pregnant women. Structural aberrations (51.96%) were more common than numerical (48.03%). The most common chromosomal abnormalities in both groups were Down syndrome from numerical aberrations and from structural inversion of chromosome 9 (Table 2).
Frequency and type of chromosomal abnormalities for the entire sample were Down
syndrome in 27 (26.47%) cases, Edwards syndrome in 7 (6.86%) cases, Patau
syndrome in 2 (1.96%) cases, Turner syndrome in 4 (3.92%) cases, other sex
chromosome abnormalities in 3 cases (2.94%), triploidy in 1 (0.98% case,
monosomy in 2 (1.96%) cases, mosaics in 3 (2.94%) cases, translocations in 2
(1.96%) cases, inversion of chromosome 9 in 39 (38.23%) cases and other
structural abnormalities of chromosomes in 10 (9.8%) cases (Table 2). There is
no statistically significant difference between the occurrence of
chromosomopathies between two examined group of pregnant women (p
The average age of the mother for all patients with proven chromosomopathies
after AC were 34.38 (
| Type of abnormalities | Total | ||
| Mean (SD) | Mean (SD) | Mean (SD) (from–to) | |
| Down syndrome | 24.66 ( |
38.87 ( |
32.78 ( |
| Edwards syndrome | 25.5 ( |
42.66 ( |
32.85 ( |
| Turner syndrome | 27.33 ( |
36 | 29.5 ( |
| Inversion of 9 chromosome | 30.45 ( |
37.75 ( |
35.93 ( |
| Other* | 28.44 ( |
38.18 ( |
34.68 ( |
| Total | 27.46 ( |
38.3 ( |
34.38 ( |
| *45, XX (1), 45, XY (1), 47, XXX (2), 47, XXY (1), Structural abnormalities of chromosomes 1 (1), 5 (1), 6 (1), 9 (1), 12 (1), 13 (1), 16 (1), 21(2), Mosaics (3), Translocations (2), Inversions (2), Sy Patau (2), Triploidy (1). | |||
Short-term complications that occurred in the period of four weeks after the intervention were spontaneous abortion in 26 (1.17%) cases, missed abortion in 4 (0.18%) cases and premature rupture of membrane in 4 (0.18%) cases.
In present study of the total number of amniocentesis (AC) performed over a period of 13 years, the largest number was performed in 2014 and since then there has been a decreasing trend in the number of performed AC. The lowest number was recorded in 2020.
The decreasing trend may be due to the availability of tests that determine the fetal DNA from the maternal blood by a non-invasive procedure [10, 11]. In recent years, a non-invasive prenatal test (NIPT) has been widely used to determine fetal DNA from maternal plasma.
However, NIPT is a screening method not thought to replace invasive testing nor in cases like increased maternal age were numbers for invasive testing has dropped. Also, the cost of these tests is too high to be used as a primary screening method in pregnant populations and can be recommended to high-risk pregnant women [11].
Other invasive procedures in prenatal diagnostics, such as cordocentesis and chorionic villus sampling, are not routinely performed in our institution. The reasons are numerous, however the leading ones are technical reasons; lack of equipment but also accompanying pathohistological laboratories, more education and routines by subspecialists in fetal medicine and obstetrics. However, chorion villus sampling (CVS) is a safe procedure performed from 11 to 13 week of pregnancy, and can be done safely after 13 weeks. CVS has the same indications as amniocentesis, and the abortion rate of 0.5% to 1% is similar to that of amniocentesis.
The most common indication for AC in a recent study was the age of the mother, aged 35 years and older. AC was performed in 1454 (65.7%) pregnant women aged 35 years and older. In a study by Turkish authors for more than twenty years, where the mother’s age of 35 years and older was an indication for AC in 48.2% of pregnant women, which is lower than in our study [12].
Anuwutnavin et al. [13] showed that maternal age (above 35 years of age) was the most common indication for AC, in 94.6% of cases. The age of the mother is a well-known risk of developing chromosomal abnormalities. Abnormal karyotype in pregnant women older than 35 years of age was found in 4.47% of cases [14].
Our results are similar to those published by Xiao et al., where 2.79% of karyotype abnormalities were recorded in pregnant women over the age of 35 [15], as well as the results of the Vičić et al. [16] study, where Down syndrome was found in 2.4% of cases. In a recent study in pregnant women with trisomy 21, in 13 cases the indications for AC were advanced patient’s age.
Due to the high rate of detection of chromosomal abnormalities, the nuchal translucency is responsible for the increase in the number of AC and the most commonly used method of fetal screening [17].
Wapner et al. [18] have shown that combined screening for trisomy 21 and trisomy 18 in the first trimester, where with maternal age, nuchal translucency, the value of PAPP-A (pregnancy associated plasma protein A) and free beta human chorionic gonadotropin is effective in clinical practice. This screening identified 85.2% of trisomy 21 cases with 9.4% false positives, 90.9% of trisomy 18 cases with false positives of 2% [18]. In our study in 11 pregnant women with trisomy 21, nuchal translucency was an indication for AC. Ultrasound examination is recommended for all pregnant women as part of routine pregnancy checks and biochemical tests in pregnant women above 35 years of age, which may reduce the number of ACs as an invasive procedure in prenatal diagnosis [17].
In a recent study, chromosome 9 inversion was found in 39 (1.68%) pregnant women, which had no effect on the karyogram. Chromosome analysis of both parents is recommended. The average age of the pregnant women was 35.93 years of age. Chromosome 9 inversion was found in a significantly higher percentage in a study conducted in Turkey, where chromosome 9 inversion was found in 21.7% of pregnant women over twenty years and chromosome analysis of both parents was also recommended [12].
Seven hundred and sixty-three (33.01%) AC was performed in pregnant women younger than 35 years of age. In this group of pregnant women, the largest number of AC was performed due to a positive family history, 227 (29.91%), and in second place is the thickness of the nuchal translucency, 147 (19.37%). A retrospective study over a thirteen-year period found that in the group of pregnant women younger than 35 years of age, the most common indication for invasive prenatal diagnosis was an abnormal ultrasound finding (60.4%), and in 30.2% amniocentesis was performed due to biochemical markers [16].
In 79 (10.41%) pregnant women AC was done because of positive biochemical markers, which is a higher number than in the study of Miyake et al. [17] where biochemical markers were indicated as an indication in 11 cases. Chromosomopathies in previous pregnancies were an indication in 41 pregnant women (5.4%), which is slightly higher than in the study in Turkey (2.7%) [12].
In present study, AC was performed in 34 twin pregnancies. All cases of mosaicism were found in twin pregnancies, in four (2.94%). In two cases, the indications for karyotyping were maternal age, in the other two cases, the indications were enlarged ventricles and cysts of the choroid plexus.
In our study, miscarriage as a complication of the AC was recorded in 1.53% of cases, higher than reported other studies in 0.2% to 0.35%, respectively [13, 19]. In our study we do not have bakcround data between two groups with and without complications, like a obesity, low PAPP-a, in vitro fertilisation, uterine fibroids, maternal age, increased nuchal translucency, etc. [19]. This differences is important, because it is more the patient than the procedure itself that promotes miscarriages and complications [19]. On pregnant woman’s decision to undergo AC influence the risk of miscarriage after the intervention. The expected rate of miscarriages is 1 in 200 cases or 0.5% [20].
Many studies show that the risk of pregnancy loss resulting from the AC is lower than previously reported [13, 19]. Following the recommendations of the American College of Obstetricians and Gynecologists, pregnancy loss is less than 1 in 300–500 cases [4], even lower in experienced hands and centers [21]. In a multicentric prospective study, 1% of miscarriages were recorded after AC, and 0.94% in the group of pregnant women in whom it was not performed [22]. A slightly higher-miscarriages rate was found in twin pregnancies after AC, 2.4% [23].
The strengths of the present study were the large sample size, a long period that the study covers and one of the largest tertiary centers in our country in which ACs were performed. The greatest strength of our study is the large data set on prenatal diagnosis, including clinical indications, maternal age, gestational age and outcome of amniocentesis, including fetal gender and the frequency and type of chromosomal abnormalities. The strength of our study is also optimal follow-up period for estimating the rate of the post procedure pregnancy loss .
The present study was limited by the lack of a control group to measure and distinguish the background and procedure-related fetal loss rate. A limitation of our study is absence of the stratification and direct correlation of AC results and complications with individual indications for AC, but this may be subject of our future study. Another limitation of the present study was that the retrospective nature of the study, a long study period that may introduce a bias, and lack some of data such as socioeconomic status and educational level. Low socioeconomic status and educational level result in an unequal access of prenatal diagnostic tests for pregnant women, but this could be the subject of our future study.
In conclusion, AC was found to be a safe invasive prenatal diagnosis method. Advanced maternal age, as expected, demonstrated the strongest association with the uptake of AC and trisomy 21 was the most prevalent abnormal finding. Ultrasound abnormalities are now the most common indication for diagnostic testing in women younger than 35 years, supporting the benefits of early fetal structural assessment. AC through conventional and molecular cytogenetic methods is essential for definite diagnosis of chromosomopathy and appropriate genetic counseling.
GG and AC designed the research study. GG and AC performed the research. AH, GB and AL provided help and advice on the experiments. GG and AC analyzed the data. All authors contributed to editorial changes in the manuscript. All authors have read and approved the final manuscript.
All subjects gave informed consent to be included before participating in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the University Clinical Center Tuzla (approval number 02-09/2-23/21).
Thanks to all the peer reviewers for their opinions and suggestions which improved the quality of our manuscript.
This research received no external funding.
The authors declare no conflict of interest.
