Objective: To investigate associations of the time
interval between hCG administration and oocyte pick up with oocyte maturation,
embryonic morphology, morphokinetics, and IVF outcome, in different age groups.
Design: A retrospective cohort study. Setting: An academic
medical center. Patients: Women who underwent IVF and ICSI cycles in
which all oocytes were incubated in a time lapse microscopy system (EmbryoScope).
Cycles were stratified by age and time from hCG exposure.
Interventions: None. Main Outcome Measures: Of 2185 IVF/ICSI
OPU cycles, 820 cycles were included in the analysis. Final analysis was made on
796 cycles (4930 oocytes). Oocyte number and their maturity, fertilization rates,
and embryo quality defined by morphology and morphokinetics,
clinical pregnancy, and live birth rates. Results: The
median hCG-oocyte pick up (OPU) interval was 34.45 hours. Among women over age 36
years, longer intervals were associated with both a higher fraction of mature
oocytes (P
The first IVF baby was born after laparoscopic retrieval of a single mature oocyte, timed by intensive monitoring of the urine LH surge in the natural cycle [1]. Various protocols for ovarian stimulation with gonadotropins combined with GnRH analogues have since been introduced, and the interval to oocyte pick up (OPU) is timed by triggering ovulation with hCG or GnRHa [2].
The interval between LH surge and ovulation allows for maturation of the oocyte and cumulus cells, and culminates in resumption of the last steps of meiosis, modulated by a complex series of biochemical events [3].
Indeed, several studies have suggested that the hCG-OPU interval has considerable impact on follicular maturation, the number of oocytes with fully expanded cumulus, metaphase II (MII) oocytes, embryo developmental competence, and IVF outcomes [4, 5, 6].
Based on literature from the 1970s and studies on patients who used clomiphene citrate (CC) or human menopausal gonadotropin (hMG) for ovulation induction, retrieving oocytes 36 hours (h) after the administration of hCG has been common practice for four decades [7, 8]. This is considered the correct time for completion of follicular development and oocyte maturation, and reduces the chances of cycle cancellation due to unexpected spontaneous ovulation [9]. However, in most IVF centers, several patients are scheduled for OPU on the same day when usually only one theatre is available. Hence, maintaining the exact 36-hour (h) interval is a challenge. Therefore, the commonly practiced interval is generally 32 to 38 h [10, 11].
Several studies that challenged the hCG-OPU interval in the past did not find consensus nor a conclusive recommendation as to the optimal interval from hCG administration to OPU. Some studies reported that a 36 h interval is inferior to a longer interval (up to 39 h) with regard to the number of mature oocytes retrieved [10, 11]. However, other studies concluded that a longer hCG to OPU interval did not have a significant impact on the outcome of IVF treatment cycles [12, 13].
Finally, the latest meta-analysis found that prolonging the interval between hCG priming and oocyte retrieval can increase the proportion of mature oocytes [14]. However, the fertilization, implantation, and pregnancy rates did not differ significantly with prolonged intervals.
Views are mixed regarding a possible impact of an association of age with a
shorter or longer hCG-OPU interval on IVF outcomes [15, 16]. A retrospective
analysis showed much higher oocyte immaturity in IVF cycles for women aged 41
years or more [15]. In 2011, Reichman et al. found no consistent
difference in oocyte yield, maturity, fertilization, or embryo quality between
cycles with a prolonged interval of
The trend to delay parenthood is enhancing around the world and the proportion of live births to older mothers 35–49 years has increased dramatically in recent years [17]. Older women undergoing IVF have lower success rates [15, 16, 17], and the present research explored a new approach to this elder population. The aim of this retrospective cohort study was to investigate associations of longer and shorter hCG-OPU time intervals with oocyte maturation, embryo morphology, morphokinetics, and IVF outcome, with a focus on patients’ age.
The study was approved by the local Institutional Review Board. Since the study was a retrospective anonymous correlative analysis, informed consent was not needed.
We assessed all IVF and ICSI cycles conducted between June 2013 and December 2017 at Carmel Medical Center, in which all embryos were incubated in a time lapse microscopy system (EmbryoScope).
Cycles were stratified according to patients’ age (
Women underwent either a long protocol treatment or a short protocol. With the long protocol women were administrated with GnRH-analogue in the midluteal phase followed by ovarian stimulation by recombinant FSH (Gonal-f; Merck Serono, Geneva Switzerland, or Puregon; Schering-Plough; Kenilworth, NJ) or hMG (Menopour, Ferring, Langley, United Kingdom) when ovarian suppression was confirmed. Whereas in the short protocol they were administrated with recombinant FSH or hMG in the early follicular phase followed by GnRH antagonist (Orgaluran 0.25 mg Merck Serono, Geneva Switzerland) from day 3 of stimulation to prevent early lutenization.
Recombinant hCG 250 mg SC (Ovitrelle; Merck Serono) was administered when at least three follicles reached a mean diameter of 18 mm on transvaginal ultrasound examination. Ultrasound-guided oocyte pickup was performed 33–37 hours later.
With the short antagonist protocol, when E2 was
 Fig. 1.
Fig. 1.Flow chart of the study population. * NON-KID-unknown implantation data, ICSI cycles resulting in a pregnancy in which more than a single embryo was transferred. ** KID-known implantation data, ICSI cycles resulting in a pregnancy in which only a single embryo was transferred and ICSI cycles did not result in pregnancy regardless of the number of embryos transferred.
Luteal support consisted of vaginal micronized progesterone (Utrogestan 800 mg/d Besin Healthcare, Belgium) or Endometrin 200 mg/day (Ferring, Israel) for 14 days, until serum BCHG level was measured to confirm pregnancy.
Oocytes were inseminated by standard IVF or ICSI procedures on metaphase II oocytes according to sperm quality. If needed for ICSI oocytes were first denudated.
Oocyte post-insemination were placed in individual micro well culture dishes
(EmbryoSlide; Unisense Fertilitech), filled with Global total (LifeGlobal, US)
universal medium. Dishes were then loaded into the EmbryoScope. Embryo culture
was performed at 37
The percentage of mature oocytes was calculated as the number of MII oocytes divided by the number of retrieved oocytes. Fertilization rate was calculated as the number of 2pn fertilized oocytes divided by the number of MII oocytes.
Embryo morphology was assessed according to a modified classification by Cummins
J et al., 1986 [18]. Briefly, embryos were graded according to three
parameters: the number of blastomeres, uneven or even cell size, and the
percentage of fragmentations. Grading was classified according to three groups:
Group 1, low quality embryos including embryos with an abnormal number of
blastomeres, unevenly sized blastomeres, and
Blastocysts were graded according to the modified Gardner grading scale of
trophoblast morphology (cohesive epithelium vs. loose epithelium and
trophectoderm), inner cell mass morphology, and blastocyst cavity size [19].
Blastocysts were graded according to three classifications: Group 1, low quality
embryos, including embryos with low morphology of the trophoblast and inner cell
mass, and small or non-existing cavity; Group 3, including high
quality embryos with high morphology of the trophoblast and inner cell mass, and
a large blastocyst cavity (
For each age and interval group, data was collected regarding treatment outcomes, including the percentage of mature oocytes retrieved, fertilization rate, the number of embryos transferred, the number of high-quality embryos, pregnancy and live births.
Each embryo was evaluated by a detailed time-lapse analysis (EmbryoScope, Unisense Fertilitech), which measured the exact timing of the developmental events in hours after insemination, as described by Meseguer M et al., 2011 [20]. Images were acquired in seven focal planes for each embryo every 15 minutes. The references for the recorded timings were the time of insemination defined by adding sperm to oocytes (IVF) or by ICSI. The terms t2, t3, t4, t5, t6, t7, and t8 refer to the respective timings of the appearance of embryos with 2, 3, 4, 5, 6, 7, and 8 well-defined blastomeres.
For the purpose of t2 calculation, defined as hours post insemination (cleavage
to 2 cells), only ICSI cycles were included; the range 25–27 h was previously
defined as the optimal time [19]. Accordingly, t2 was divided into 3 intervals:
under 25 h, 25–27 h, and
Primary outcome: We examined associations of the hCG to OPU interval with the number and percentage of mature oocytes according to women’s age, embryo quality as graded by morphology and timing of the first cleavage (t2).
Secondary outcomes: Clinical pregnancy rates (CPR) and live birth rates (LBR) achieved at different hCG to OPU intervals in relation to women’s age.
Statistical analysis was performed using IBM statistics (SPSS) vs. 24. The continuous variables are presented as means and standard deviations or medians and IQR. The categorical variables as percentages.
hCG-OPU interval was divides into two categories according to the median.
Differences in demographical & clinical characteristics between the 2 levels of hCG-OPU interval were analyzed using the Chi square test for the categorical variables and Independent t-test or Mann-Whitney, as appropriate, for the continuous variables.
T2 cells were divided into 3 levels and P for trend was calculated to evaluate the association with clinical pregnancy.
P
Of 2185 IVF/ICSI OPU cycles, 820 cycles were included in the analysis. They yielded 5086 oocytes post insemination, which were incubated in a time lapse microscopy prior to embryo transfer. The main inclusion criteria was incubation of all post insemination oocytes in a time lapse microscopy. There was no exclusion of patients due to the infertility cause. Final analysis was made on 796 cycles (4930 oocytes) which ended in fresh embryo transfer (Fig. 1).
The mean duration of hCG to OPU interval was 34.74
The median time calculated for the hCG to OPU interval was 34.45 hours, the
range was (33.45–38.45). Two groups were determined according to the median
interval duration: retrieval
The mean age, number of oocytes retrieved, percentage of mature oocytes,
fertilization rates, number of embryos transferred, embryo grading and
morphokinetics, CPR, and LBR were collected and compared between the two interval
groups (
| hCG-OPU intervals | P-value | ||
| n = 426 | n = 370 | ||
| Age, mean |
35.0 |
35.1 |
0.739 |
| Number of mature oocytes (MII) | 6.1 |
6.3 |
0.421 |
| % Mature oocytes | 77.0 |
79.3 |
0.033 |
| Number of oocytes retrieved | 8.3 |
8.2 |
0.857 |
| Fertilization rate | 68.2 |
65.8 |
0.178 |
| Number of embryos transferred (median) | 2 (1; 2) | 2 (1; 2) | 0.284 |
| CPR (%) | 123 (28.9) | 107 (28.9) | 0.994 |
| LBR (%) | 98 (23.1) | 87 (23.5) | 0.880 |
| Embryo grading and morphokinetics | |||
| hCG-OPU intervals | P-value | ||
| Embryo grading | n = 1016 | n = 793 | |
| Group 2 + 3 (%) | 822 (80.9) | 667 (84.1) | 0.076 |
| Group 1 (%) | 194 (19.1) | 126 (15.9) | |
| hCG-OPU intervals | P-value | ||
| Number of embryos | n = 635 | n = 496 | |
| t2 |
161 (25.4) | 137 (27.6) | 0.611 |
| t2 25–27 (%) | 152 (23.39) | 121 (24.4) | |
| t2 |
322 (50. 7) | 238 (48.0) | |
| MII, metaphase II; CPR, clinical pregnancy rate; LBR, live birth rate. | |||
After analyzing the data forthe entire research population referring the age as
a continuous parameter, we observed a statistical difference between the two
groups (age less than 36 years and over 36 years old) comparing the results in
the two interval groups (
For women aged
| hCG-OPU intervals | P-value | ||
| n = 227 | n = 183 | ||
| Number of mature oocytes (MII) | 6.8 |
6.9 |
0.667 |
| Number of oocytes retrieved | 9.1 |
9.2 |
0.609 |
| % Mature oocytes | 78.0 |
78.0 |
0.688 |
| Fertilization rate | 65.3 |
61.4 |
0.150 |
| Number of embryos transferred (median) | 2 (1; 2) | 2 (1; 2) | 0.268 |
| CPR (%) | 81 (35.8) | 66 (36.1) | 0.962 |
| LBR (%) | 70 (31.0) | 55 (30.1) | 0.841 |
| Embryo grading and morphokinetics | |||
| hCG-OPU intervals | P-value | ||
| Embryo grading | n = 551 | n = 397 | |
| Group 2 + 3 (%) | 452 (82.0) | 327 (82.4) | 0.894 |
| Group 1 (%) | 99 (18.0) | 70 (17.6) | |
| hCG-OPU intervals | P-value | ||
| Number of embryos | n = 386 | n = 303 | |
| t2 |
102 (26.4) | 92 (30.4) | 0.341 |
| t2 25–27 (%) | 91 (23.6) | 76 (25.1) | |
| t2 |
193 (50.0) | 135 (44.6) | |
| MII, metaphase II; CPR, clinical pregnancy rate; LBR, live birth rate. | |||
For women over age 36 years (n = 386), a longer interval duration (
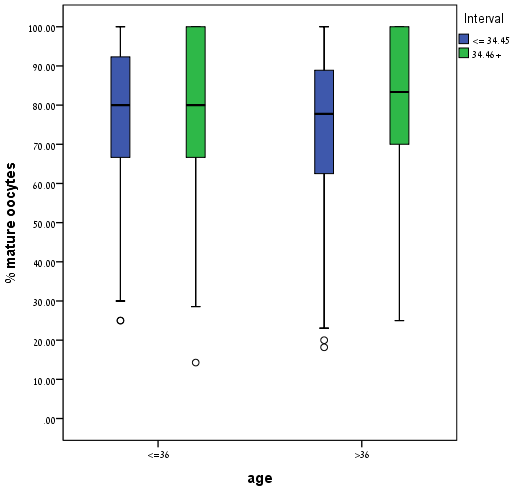 Fig. 2.
Fig. 2.Percentage of mature oocyte according to women’s age at different hCG-OPU intervals. This figure illustrates the percentage of mature oocytes in women aged
| hCG-OPU intervals | P-value | ||
| n = 199 | n = 187 | ||
| Number of mature oocytes (MII) | 5.4 |
5.7 |
0.331 |
| Number of oocytes retrieved | 7.33 |
7.2 |
0.989 |
| % Mature oocytes | 75.9 |
80.6 |
0.008 |
| Fertilization rate | 65.2 |
64.1 |
0.580 |
| Number of embryos transferred (median) | 2 (1; 3) | 2 (1; 2) | 0.386 |
| CPR (%) | 42 (21.1) | 41 (21.9) | 0.845 |
| LBR (%) | 28 (14.1) | 32 (17.1) | 0.410 |
| hCG-OPU intervals | P-value | ||
| Embryo grading | n = 465 | n = 396 | |
| Group 2 + 3 (%) | 370 (79.6) | 340 (85.9) | 0.016 |
| Group 1 (%) | 95 (20.4) | 56 (14.1) | |
| hCG-OPU intervals | P-value | ||
| Number of embryos | n = 249 | n = 193 | |
| t2 |
59 (23.7) | 45 (23.3) | 0.942 |
| t2 25–27 (%) | 61 (24.5) | 45 (23.3) | |
| t2 |
129 (51.8) | 103 (53.4) | |
| MII, metaphase II; CPR, clinical pregnancy rate; LBR, live birth rate. | |||
| hCG-OPU intervals | P-value | ||
| n = 102 | n = 93 | ||
| Number of oocytes retrieved | 6.3 |
6.5 |
0.795 |
| Number of mature oocytes (MII) | 4.6 |
5.3 |
0.197 |
| % Mature oocytes | 75.0 |
82.47 |
0.014 |
| Fertilization rate | 69.9 |
64.7 |
0.145 |
| Number of embryos transferred (median) | 2 (1; 3) | 2 (1.5; 2) | 0.301 |
| CPR (%) | 16 (15.7) | 15 (16.1) | 0.933 |
| LBR (%) | 10 (9.8) | 13 (14.0) | 0.367 |
| Embryo grading and morphokinetics | |||
| hCG-OPU intervals | P-value | ||
| Embryo grading | n = 229 | n = 184 | |
| Group 2 + 3 (%) | 183 (79.9) | 159 (86.4) | 0.082 |
| Group 1 (%) | 46 (20.1) | 25 (13.6) | |
| hCG-OPU intervals | P-value | ||
| Number of embryos | n = 113 | n = 81 | |
| t2 |
23 (20.4) | 16 (19.8) | 0.963 |
| t2 25–27 (%) | 26 (23.0) | 20 (24.7) | |
| 64 (56.6) | 45 (55.6) | ||
| t2 |
23 (20.4) | 16 (19.8) | 0.963 |
| MII, metaphase II; CPR, clinical pregnancy rate; LBR, live birth rate. | |||
Outcomes stratified as trigger less than 35 hours trigger 35–37 hours, and trigger more than 37 hours is presented in Table 6.
For women aged
| hCG-OPU Interval | ||||||||
| t2 intervals | 25–27 | P | 25–27 | P | ||||
| All patients | n = 77 | n = 73 | n = 190 | n = 77 | n = 59 | n = 139 | ||
| CPR (%) | 10 (13.0) | 5 (6.8) | 4 (2.1) | P |
12 (15.6) | 4 (6.8) | 8 (5.8) | P = 0.019 |
| n = 47 | n = 36 | n = 101 | n = 45 | n = 34 | n = 72 | |||
| CPR (%) | 8 (17.0) | 5 (13.9) | 3 (3.0) | P = 0.003 | 8 (17.8) | 4 (11.8) | 7 (9.7) | 0.212 |
| n = 30 | n = 37 | n = 89 | n = 32 | n = 25 | n = 67 | |||
| CPR (%) | 2 (6.7) | 0 | 1 (1.1) | 0.115 | 4 (12.5) | 0 | 1 (1.5) | 0.018 |
| CPR, clinical pregnancy rate; LBR, live birth rate; KID, known implantation data. t2: the time to the appearance of embryos with 2 well-defined blastomeres. * t2 results for KID embryos in ICSI cycles, only SET for positive KID, all embryos transferred included for negative KID. | ||||||||
| hCG-OPU intervals | 35–37 | P | ||
| N = 482 | N = 311 | N = 3 | ||
| Mean age | 34.9 |
35.2 |
35.8 |
0.970 |
| Number of oocyte retrieved | 8.3 |
8.1 |
8.3 |
0.930 |
| % mature oocytes | 76.7 |
80.0 |
87.9 |
0.031 |
| fertilization rate | 63.6 |
62.1 |
78.3 |
0.388 |
| Number of embryos transferred | 1.9 |
1.8 |
2.3 |
0.163 |
| % pregnancies | 143 (29.7) | 86 (27.7) | 1 (33.3) | 0.813 |
| % deliveries | 114 (23.7) | 70 (22.5) | 1 (33.3) | 0.889 |
| Embryo grading (%) | 0.505 | |||
| Group 1 + 2 | 437 (90.9) | 288 (92.9) | 3 (100) | |
| Group 3 | 44 (9.1) | 22 (7.1) | 0 |
Morphokinetic analysis of KID embryos at the two hCG-OPU intervals, stratified
by age groups (
Our present study found that in women aged
The LH surge promotes the activation of multiple signaling networks in the ovarian follicle, which leads to the final steps of meiosis and oocyte maturation, as well as differentiation of granulosa cells and cumulus cells around the oocyte [11]. Based on the natural cycle physiology, the period of time that oocytes mature in vivo, which is represented by the interval from hCG administration to OPU, has been considered to have a predominant effect on the success of IVF, either by in vitro insemination or ICSI [9, 19].
Although the gold standard for that interval was identified as 36 hours by several studies, we found that in daily practice with many oocyte retrievals on the same day, keeping an accurate 36 hours interval is often challenging. Indeed, over the years, other studies reported the use of a wide range of intervals, 32–41 hours [9].
Most studies analyzing hCG to retrieval interval generally did not stratify success in relation to age. In a study by Mansour RT et al. 1994, 90 couples with male factor infertility undergoing ICSI cycles were randomized to 35-, 36-, and 37-hour hCG interval to retrieval. They found significantly more metaphase II oocytes at 36 and 37 intervals. However, the 30 patients who underwent delayed retrieval were on average 2.2 years younger than their 36-hour counterparts.
Bjercke et al., 2000 published a study in which 170 patients were randomized to either 34- or 38-hour retrieval intervals in IVF cycles and found no significant differences in outcomes. Interestingly, women in the delayed retrieval arm (38 h interval) were on average 2 years younger than those who underwent retrieval at 34 hours. The authors concluded that the interval from hCG injection to retrieval could fall anywhere within the 4-hour time interval included in their study, with no need for adherence to strict rules for retrieval times.
In 2001, Nargund et al., enrolled 533 consecutive patients who were randomly allocated to times for oocyte collection that ranged from 33 to 41 hours. An upward trend in the rate of clinical pregnancies was observed as the time interval increased; however, no statistically significant results were obtained.
Interestingly, none of the 533 women for whom retrieval was as late as 41 hours had ovulated before retrieval. Reichman et al. reported similar results regarding age-factor related effects and showed favorable results by extending the hCG-OPU interval in women over the age of 40 years [16].
Bosdou et al., 2015 enrolled 156 normo-ovulatory women which were randomized to have oocyte retrieval performed 36 h (n = 78) or 38 h (n = 78) following hCG administration. No significant differences were observed between the groups yet, woman’s age in the multivariable analysis performed was negatively associated with oocyte retrieval rate.
Our findings concur with those of a 2011 meta-analysis [21] that reported an increased percentage of mature (MII) oocytes following prolonging the interval between hCG priming and oocyte retrieval; however, that analysis did not stratify by age [14]. In the present study, we set out to investigate a possible benefit, in terms of the number of mature oocytes and embryonic morphology, according to age groups, when the duration of patient exposure to hCG was longer.
We analyzed oocyte maturation separately in ICSI cycles in which we could accurately define the percentage of MII oocytes after denudation of cumulus cells.
The primary outcomes of the current study were the number and % of retrieved mature oocytes and embryo quality at different intervals, in relation to women’s age. This was based on our prior observations that older women seemed to benefit from a delay in oocyte retrieval, and on other reports that showed that oocyte immaturity tended to increase with increasing women’s age [15].
We analyzed the data according to the median interval of hCG trigger to OPU at our center (34.45 h); this reflects deviations that occurred in our work routine. Such variance made it possible to investigate whether outcomes might be optimized at different retrieval times. No guidelines are currently available that advise prioritizing and scheduling women individually according to age.
Even dough the prolonged interval could not increase the fertilization rate,
pregnancy rate and live birth rate, our results show that women aged
Our results demonstrated in the general population, a positive association of
the hCG-OPU interval with the percentage of mature oocytes retrieved. Moreover,
among women
We are aware to the low mean number of retrieved oocytes, fertilization rate and clinical pregnancy rate. This may be partially explained by heterogeneity of the study population and a relative high percentage of poor responders.
In the current study, all embryos were incubated and assessed in the time laps microscopy. The use of this technology enabled standardizing embryo assessment in all IVF/ICSI cycles. Regarding embryo morphokinetics and treatment results, we assessed relationships of age with different interval groups, cleavage timing to two cell embryos (t2), and treatment outcomes.
The results show no correlations of women’s age, the hCG-OPU interval, and the
t2 with the outcomes assessed, namely pregnancy rate and live birth rates per
transferred embryo. However, among all patients, regardless of age or hCG
interval, those with a higher number of embryos with cleavage time
Nowadays, seeking motherhood is often postponed, and a tailored medical regime
is the gold standard, by which protocols are often adjusted to specific
populations. Our findings suggest that scheduling our patients for OPU should
prioritize older women, who may particularly benefit from a longer hCG-OPU
interval (
Our study is not without limitations. Although we showed that delaying retrieval past 34.45 hours can result in favorable outcomes in older women, it is not yet clear whether there is an upper limit to this interval. Moreover, studies with larger numbers of women at each of the intervals will afford greater statistical power and a more precisely defined interval. The biological mechanism to explain our results is also an interesting subject for further study.
In conclusion, our results show that flexible hCG-OPU intervals of 33–37 hours
(median 34.4 h) resulted in comparable embryo quality and IVF outcome in the
younger population
SS drafted the article, performed literature search and conceived methodology with DM. BL and KM assisted in drafting parts of the article. DM, LBS and WMZ edited the final version of the article. DM performed extensive editing of the primary draft. All authors have approved the final version of the article.
This prospective study was approved by the Institutional Review Board. As a prospective article there was no intervention made with patient’s treatment and no consent to participate needed.
We thank MS. Yuval Rita for technical assistance.
This research received no external funding.
The authors declare no conflict of interest.
