Background: Carcinosarcoma is a rare malignancy of the female reproductive system, consisting of carcinomatous and sarcomatous components. It originates from the paramesonephric and mesonephric duct residues and is also called malignant mixed Mullerian tumor. Case Presentation: A 58-year-old post-menopausal woman, gravida 2, parity 2, who had no known diseases and no previous surgery presented to another clinic with pelvic pain that had been persisting for about a year and vaginal bleeding for the past month. There was continuous bleeding that was not associated with coitus, and cervical biopsy was reported as carcinosarcoma of heterologous type (the epithelial part was serous carcinoma and the mesenchymal part was chondrosarcoma); the patient was referred to our clinic and underwent evaluation. Conclusion: Effective follow-up is very important considering the aggressive clinical picture of carcinosarcoma cases. It should be kept in mind that metastasis may occur, especially in cases with hypercalcemia and deterioration of the general condition.
Carcinosarcoma is a rare malignancy of the female reproductive system, comprising carcinomatous and sarcomatous components. It originates from the paramesonephric and mesonephric duct residues and is also called malignant mixed Mullerian tumor [1]. Although the uterine corpus is the most common localization, carcinosarcomas may originate from the uterus, cervix, ovary, fallopian tube, vagina, peritoneal surfaces and all tissues of mesonephric origin [2]. Cervical carcinosarcomas are rare and fewer than 70 cases have been reported in the English literature [1]. While most cervical carcinosarcomas have homologous mesenchymal components, the presence of a heterologous mesenchymal component is limited to eleven cases in the literature [3]. We also aimed to present and discuss the case of the twelfth cervical carcinosarcoma with heterologous component that had been operated in our clinic, along with the literature. Carcinosarcoma is an aggressive tumor and its recurrence occurs in a short time after treatment. When the literature was investigated, it was found that most of the cases were at an early stage, as in our case [4, 5, 6, 7]. It was also observed that one case relapsed as early as 11 months after treatment and it was reported that the other two cases died within 7 months (Table 1).
| Patient number | Age | Stage (FIGO) | Recurrence | Death | Follow-up/overall survival (month) |
| 1 |
65 | 1b | NO | NO | 23 |
| 2 |
37 | 1b | YES | NO | 11 |
| 3 |
40 | 1b | NO | NO | 28 |
| 4 |
73 | 1b | NO | NO | 36 |
| 5 |
39 | 1b | NS | YES | 74 |
| 6 |
54 | 2a | NS | YES | 7 |
| 7 |
54 | 1b | NO | NO | 13 |
| 8 |
62 | 4b | YES | NO | 36 |
| 9 |
63 | 2a | NO | NO | 10 |
| 10 |
59 | 3b | NO | NO | 4 |
| 11 |
64 | 2a | NS | YES | 7 |
| 12 (new case) | 58 | 1b3 (FIGO 2018) | YES | NO | 20 |
| NS, not state. | |||||
A 58-year-old post-menopausal woman, gravida 2, parity 2, who had no known
diseases and no previous surgery presented to another clinic with pelvic pain
that had been persisting for about a year and vaginal bleeding for the past
month. There was continuous bleeding that was not associated with coitus, and
cervical biopsy was reported as carcinosarcoma of heterologous type (the
epithelial part was serous carcinoma and the mesenchymal part was
chondrosarcoma); the patient was referred to our clinic and evaluated. It was
learnt that the cervical cytological examination of the case had never been
performed. In the speculum examination, there was a large cervix according to age
with multiparosis and hemorrhage, in addition to erosion. In the bimanual
examination, the parameters were free of tumor, and a mass with a smooth surface
was detected. On transvaginal ultrasonography, a solid mass of 9
 Fig. 1.
Fig. 1.Uterine cervical mass on magnetic resonance examination (sagittal and coronal section).
The operation was completed by performing type 2 radical hysterectomy, bilateral
salpingo-oophorectomy, bilateral pelvic and paraaortic lymph node dissection and
omentum biopsies. The final pathology of the case was reported to be of cervical
origin; the epithelial part was serous carcinoma and the mesenchymal part was
chondrosarcoma and osteosarcoma with heterologous component carcinosarcoma
(Fig. 2). The left ovarian mass was reported to be a mature cystic teratoma.
The largest size of the tumor was 10 cm; it did not extend to the vagina; there
was no tumor at the vaginal surgical margin, the omentum was benign, the lymph
nodes and the cytology were negative, but the tumor was reported to have
lymphovascular invasion. In the multi-disciplinary meeting, 4 cycles of
chemotherapy (carboplatin-paclitaxel) and 28 days of external radiotherapy (28
days ERT) were planned. The patient’s CT, which was performed during the routine
controls approximately 14 months after the last dose of chemotherapy,
demonstrated opacities suggesting recurrence in the lung parenchyma, which were
evaluated by PET/CT. Positron emission tomography (PET/CT) demonstrated
multiple metastatic nodules (SUVmax: 19.3) of approximately 2.5 cm in both lungs.
In addition, a necrotic hypermetabolic mass (SUVmax: 14, metastatic lymph nodes)
and lytic metastatic lesion on the right side of L4 vertebral corpus (SUVmax: 16)
were displayed in the left paraaortic region. Treatment with 3 cycles of
cisplatin-doxorubicin was begun. PET/CT was performed after the third cycle.
Upon observation of a partial response compared to the pre-treatment findings
lung metastatic nodule (SUVmax: 19,3
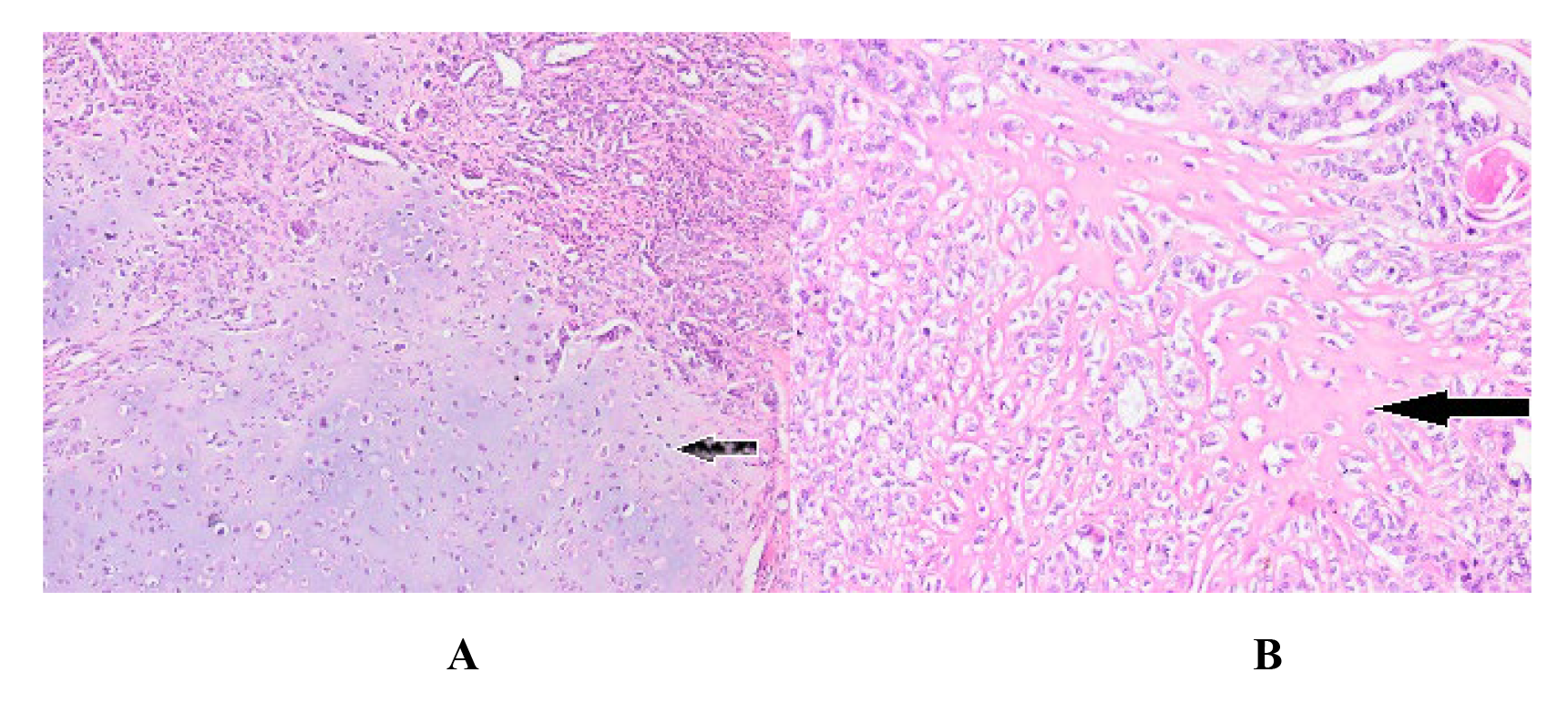 Fig. 2.
Fig. 2.Chondrosarcoma and osteosarcoma with heterologous
component carcinosarcoma on pathological examination (H&E
These tumors, defined as malignant mixed Mullerian or mesodermal tumors, have mixed histological findings of sarcoma and carcinoma. The tumor is thought to originate from totipotent mesenchymal stromal cells [8, 9, 10, 11]. The carcinomatous part is mostly glandular and the sarcomatous part may resemble the normal uterine stroma (homologous component) or non-genital tissues such as cartilage, fat, bone and smooth muscle tissue (heterologous component). Nowadays, these mixed tumors are considered to be monoclonal cell-derived malignancies that exhibit sarcomatous metaplasia, unlike the mixture of carcinoma and sarcoma [12, 13, 14].
With regard to risk factors, clinical symptoms and recurrence rates in
particular, these tumors have similar features to high-grade endometrium cancer
[13, 14]. Carcinosarcomas are seen in 3% of uterine malignancies, but in
particular, most are of uterine corpus origin; those of cervical origin are very
rare, but their prognosis is much worse [15]. The clinical prognosis is
determined by the carcinomatous component, which is most frequently high-grade
serous carcinoma [14, 16]. Almost all carcinosarcomas are seen after menopause and
the median age of the patients is 62. It is more common in the black population
and comorbidities such as obesity, diabetes and hypertension often accompany the
disorder. 7–37% of the cases have a history of pelvic radiation [12, 15].
Carcinosarcomas generally have a soft and polypoid structure that quickly fills
the area of origin and includes necrosis and bleeding areas. It can invade all
tissues at various degrees. Areas in which the spread of the tumor is most
frequently observed include the pelvis, lymph nodes, peritoneal cavity, lungs and
the liver, and it can spread to all areas of the body. This aggressive metastatic
pattern explains the poor prognosis. In the literature, it has been reported that
these cases are most common in the post-menopausal period and that the most
common clinical presentation is vaginal bleeding [17, 18]. Our case was also in
the post-menopausal period and the reason for presentation was vaginal bleeding
that had been continuing for one month. Furthermore, similar to patients
presenting with acute abdominal findings due to tumor rupture in the literature
[19], there was persisting pelvic and abdominal pain in our case, and it was
observed that there had been several emergency presentations and that treatments
had been given considering urinary tract infection. In postmenopausal period in
particular, it is important to evaluate the cases that describe vaginal bleeding
and pelvic pain for carcinoma etiology. The prognosis of carcinosarcoma is much
better when it is detected at an early stage. However, in this case, due to
advanced age, large mass and lymphovascular invasion, recurrence occurred in
multiple areas at the 14
Surgery is the main treatment in all carcinosarcomas. Total extrafascial
hysterectomy and bilateral salpingo-oophorectomy constitute the standard surgical
procedure [3]. As with other gynecological malignancies, cytology and multiple
biopsies from suspicious locations are part of the surgical staging.
Cytoreduction, lymphadenectomy, omentectomy and fertility sparing surgery are
controversial issues. There are studies showing that adjuvant radiotherapy
reduces the local recurrence, especially in early stage carcinosarcoma, but its
effects on survival are not clear [21]. Many different studies have found that
radiotherapy has no effect on survival in genital carcinosarcomas [22, 23]. The
effect of adjuvant RT in early stage carcinosarcoma cases was investigated in a
prospective study of EORTC (European Organization for Research and Treatment of
Cancer Gynecological Cancer Group Study) with other uterine sarcomas. Adjuvant
radiotherapy was found to be useful in local control of the disease when the
analysis was performed for patients with carcinosarcomas, but the same benefit
was not determined for disease-free survival or overall survival [24]. Our
patient was given 28 days of radiotherapy treatment, but multiple relapses were
detected in the lungs and bones at the end of the treatment; therefore, it is
difficult to say anything about the effect of radiotherapy on disease-free
survival. When the literature is investigated, the information on adjuvant
chemotherapy in carcinosarcoma is controversial; most of the data are based on
non-randomized-controlled studies and there is no clear chemotherapeutic agent
combination. Furthermore, the effects of these combinations on disease free
survival and overall survival are not clear [20]. In our case, the combination of
4 cycles of carboplatin + paclitaxel was used in adjuvant therapy, and upon
detection of multiple relapses, the chemotherapy regime of the patient was
switched to cisplatin + doxorubicin, which has been featured in some studies
[25]. The radiotherapy option can be used in patients who cannot be operated due
to concomitant disease, poor performance and advanced age, and hormonal treatment
options can be applied in patients with extensive metastatic disease [26, 27]. In
the case of Ribeiro B et al., despite carboplatin + paclitaxel
chemotherapy with external radiotherapy after radical surgery, widespread bone
metastases were detected on the PET-CT (positron emission tomography) performed
due to the presence of weakness and hypercalcemia at the
5
Effective follow-up is very important considering the aggressive clinical picture in carcinosarcoma cases. It should be kept in mind that metastasis may occur, especially in cases with hypercalcemia and poor general condition. When the literature is investigated, it is seen that our case is the twelfth carcinosarcoma case of cervical origin with heterologous component. Prognostic factors are still uncertain in cervical carcinosarcomas and due to its rarity, there is no consensus for the optimal treatment. However, considering the general aggressive behavior of carcinosarcomas, the choice should be towards aggressive surgical treatment.
OB, MSB, CK, CD and TS assembled, analyzed and interpreted the patient data. All authors contributed to writing the manuscript. All authors read and approved the final manuscript.
The patient gave her informed consent for redaction and publication of the case report. The report was approved by Hospital Akdeniz University’s Ethics Committee for publication. Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
We thank the patient and her family for allowing us to share her details and thank all the staff of Nyala Sudan Turkey Training and Research Hospital and Akdeniz University Hospital.
This research received no external funding.
The authors declare no conflict of interest.
The authors agree to make the raw data and materials described in our manuscript freely available.
