koreadryoo@gmail.com (Jeong-Eun Yoo)
†These authors contributed equally.
Purpose of Investigation: Ovarian aging remains a difficult
problem in infertility treatment. The traditional oriental herbs have been widely
used for female infertility treatment. This study investigated the effect of
seven oriental herbs mixture (Jogyeongbohyeoldan, JBD) on ovarian aging and
oocyte quality in aged and premature ovarian failure female mice.
Materials and Methods: C57BL/6 female mice aged 12 months (natural
ovarian aging, NOA) were administered once daily with JBD of 15 mg/kg (n
Ovarian aging remains a difficult problem in infertility treatment. It is characterized by a reduction in oocyte quality and the number of ovarian follicles [1]. The reasons for age-related decline in oocyte quality can be explained in several ways. The first is an increase in the incidence of oocyte aneuploidy, which rises abruptly in the mid-30s and reaches 80% by the age of 45 [2]. Aneuploid oocytes can result in aneuploid embryos, miscarriage, and congenital disorders at birth of an infant [3].
Second, an increase in oxidative stress within the oocytes causes age-related decline in oocyte quality. The relationship between oxidative stress and oocyte quality deterioration has been well-studied, and the primary cause for age-related decline in oocyte quality is the accumulation of mitochondrial dysfunction, which causes oxidative stress [4, 5, 6]. Several studies have reported that antioxidants such as melatonin and coenzyme Q10 can improve mitochondrial function and rescue fertility during reproductive aging by protecting ovarian reserve against oxidative damage [7, 8, 9, 10]. Inflammatory aging is a new concept that refers to a chronic and low-degree proinflammatory state which occurs with increasing age. Recently many studies have reported a significant association between inflammatory aging and the pathogenesis of premature ovarian insufficiency (POI) [11].
The third is a defect in ovarian angiogenesis, which plays an important role in a series of events of folliculogenesis, such as follicular growth and the selection of dominant follicle [12, 13, 14]. The vasculature of the follicle is needed to deliver hormones, hormone precursors, oxygen, and nutrients. Thus, an active blood supply is essential for the induction of good quality oocytes [15], and the activation of ovarian angiogenesis has been reported to improve oocyte quality and ovarian function [16, 17, 18]. Recently, it has been reported that oocyte quality can be improved by activating early follicular development, such as primordial follicle [19, 20], or ovarian microenvironment including ovarian angiogenesis during follicular development [18, 21, 22]. However, there is no a practically effective treatment to overcome ovarian aging and to improve oocyte quality.
Many studies for the effect of herbal medicine on anti-aging have been attempted for a long time [23]. Several traditional Chinese medicinessuch as Yifuning have been reported to postpone ovarian aging by antioxidant mechanisms [24]. Si-Wu-Tang (SWT) is one of traditional oriental medicines and consists of Rehmanniae Radix, Angelica Radix, Chuanxiong Rhizoma, and Paeoniae Radix. It has been widely used in the treatment of female infertility and gynecological disorders such as irregular menstrual cycle, dysmenorrheal, uterine bleeding, climacteric syndrome, and other estrogen-related diseases [25, 26]. It has also the antioxidative, anti-inflammatory, and antiaging activities [27, 28]. Astragali Radix has also the antioxidant effects [29], and CyperiRhizoma and Astragali Radix are widely used in the treatment of female infertility such as polycystic ovarian syndrome [30]. AtractylodesRhizomadhas been reported to exert antioxidation activity [31]. However, there are no studies for the effect of each herb on ovarian function or ovarian aging. In this reason, our study group was first formulated Jogyeongbohyeoldan (JBD) using SWT and these three herbs to examine the effect of the mixture of these herbs on ovarian function or ovarian aging. Traditional oriental medicines are characterized by the multicomponent/multitargeting principle and the combination of various components exhibits a synergisitic medicial effect through complementary action, neutralizing action, facilitatingaction and pharmacokinetic potentiation [32]. The structure of a formula contains four parts, namely: the chief, the deputy, the assistant(s) and the envoy. Each of these has specific functions in the formula [33]. Based on this principle, the JBD were composed of SWT as the main component, CyperiRhizoma as the deputy component, and Astragali Radix and Atractylodes Rhizomad as the assistant component, and the ratio was combined at 8 : 4 : 3 : 3, respectively.
Therefore, this study designed to investigate the effect of JBD consisting of 7 kinds of oriental herbs on ovarian aging and oocyte quality in aged and premature ovarian failure female mice. Also this study investigated the effect of JBD on expression of genes associated with the activation of primordial follicles, ovarian stem cells, and angiogenesis in the ovary.
This study is designed as a controlled experimental study using laboratory animals. All animal experiments were conducted under the guidance for the Care and Use of Laboratory Animals, approved by the Pusan National University Hospital Institutional Animal Care and Use Committee (PNUH-2019-148).
JBD was obtained from Korean Medicine Hospital of Daejeon University, and it was
manufactured at KB outsourcing preparing facility (Seoul, Korea). The formula is
composed with seven herbal plants, and the plants were identified by a botanical
expert working at KB Co. (Table 1). Briefly, the plants (720 g) were extracted
with 45 liter of distilled water for 10 hours at 100
| Herbal name | Pharmacognostic Name | Dose (%) |
| Cyperus Rhizome | CyperiRhizoma | 160 g (22.2) |
| Astragalus Root | Astragali Radix | 120 g (16.7) |
| Atractylodes Rhizome White | AtractylodisRhizoma Alba | 120 g (16.7) |
| Prepared Rehmannia Root | Rehmanniae radix preparata | 80 g (11.1) |
| Angelica Gigas Root | AngelicaeGigantis Radix | 80 g (11.1) |
| Cnidium Rhizome | CnidiiRhizoma | 80 g (11.1) |
| Peony Root | Paeoniae Radix | 80 g (11.1) |
| Total | 720 g (100) |
TOV21G cells were seeded at a 1
This study used inbred C57BL/6 mice aged 12 months as a NOA model because the reproductive life span of laboratory mice including C57BL/6 strain is significantly shorter at 7-8 months [34] and C57BL/6 mice within the 11-12 month age range are near the end of their reproductive lifespan [35]. POF model was made by the following method using anticancer drug, cisplatin. C57BL/6 female mice aged 6 to 8 weeks were administered 2 mg/kg cisplatin (Sigma-Aldrich, St Louis, MO, USA) for 10 days. Controls were injected with equal volume of saline for the same periods. Cisplatin were prepared in 0.9% NaCl (w/v) solution. The general condition and body weight of the mice were measured every day.
All mice used in the experiment were purchased from the Koatech Inc.
(Gyunggi-do, Korea) and bred under a 12 hours light/dark cycle with free access
to water and foodin an animal facility of SPF class with a temperature of 21
NOA female mice aged 12 months were weight matched and randomized into three
groups (n
The day after the final treatment of JBD to NOA mice, mice were sacrificed by
cervical dislocation. And then, both ovaries were isolated and fixed in 4%
paraformaldehyde at 4
The day after the final administration of JBD to NOA mice, both ovaries were
collected and mRNA expressions of genes associated with Hippo signaling pathway
[mammalian STE20-like protein kinase 1 (MST1), large tumor suppressor (LATS1)],
PI3K/mTOR signaling pathway [eukaryotic initiation factor 4E (eIF4E) binding
protein 1 (4E-BP1), ribosomal protein S6 kinase beta-1 (S6K1), ribosomal protein
S6 (RPS6)], ovarian stem cells (Oct4), angiogenesis [vascular endothelial growth
factor (VEGF), visfatin, and stromal cell derived factor-1
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer’s protocol. Complementary DNA (cDNA) was
synthesized from 1
| Gene name | Sequence (5’→3’) | NCBI reference number | product size (bp) |
| 4EBP1 | F: CTAGCCCTACCAGCGATGA G | NM_004095 | 109 |
| R: CCTGGTATGAGGCCTGAATG | |||
| S6K1 | F: GTAGTCCACGAACACCTGTC | NM_001114334 | 99 |
| R: TGAGGATTTGCCGTGCTGG | |||
| RPS6 | F: GGACGCTAATCTCAGTGTTCTC | NM_001374707 | 101 |
| R: CCTGGGCTTCTTACCTTCTTTG | |||
| MST1 | F: GCACAGCCATCAAAATGGT | XM_006526827 | 103 |
| R: GGGATGCTGCAACAGCTGAG | |||
| LATS | F: CAAACAGGGGCTTCTGCTGC | NM_010690 | 80 |
| R: GACTGTTCCTGTTGGGCACC | |||
| 4-Oct | F: CTTTGGCACCCCAGGCTATG | NM_001252452 | 117 |
| R: GTGAAT GCATGGGAGAGCCC | |||
| VEGF | F: CTGCTCTCTTGGGTGCACTG | NM_001025250 | 91 |
| R: CTGCTCTCCTTCTGCGTGG | |||
| Visfatin | F: CTTGTTCAGTCCTGGTATCC | NM_021524 | 148 |
| R: GCGAAGAGACTCCTCTGTAA | |||
| SDF-1α | F: GTCTAAGCAGCGATGGGTC | NM_001012477 | 92 |
| R: GAATAAGAAAGCACACGC | |||
| IGF-1 | F: GGCATTGTGGATGAGTGTTG | NM_001111274 | 82 |
| R: CTGAGTCTTGGGCATTGCGTCAG | |||
| GAPDH | F: TCAACGGCACAGTCAAGGC | NM_001289726 | 105 |
| R: CTCCACGACATACTCAGCAC | |||
GAPDH: glyceraldehyde 3-phosphate dehydrogenase, 4EBP1: eukaryotic initiation factor 4E (eIF4E) binding protein 1, S6K1: ribosomal protein S6 kinase beta-1, RPS6: ribosomal protein S6, MST1: mammalian STE20-like protein kinase 1, LATS: large tumor suppressor, OCT4: octamer-binding transcription factor 4, VEGF: vascular endothelial growth factor, SDF-1α: stromal cell derived factor-1α, IGF-1: insulin-like growth factor-1. | |||
In the second experiment, another 21 NOA and 21 POF mice were treated with JBD
in the same methods as above. The day after the last treatment of JBD, the mice
were superovulated by intraperitoneal injection with 0.1 mL of 5 IU pregnant
mare’s serum gonadotropin (PMSG; Sigma-Aldrich) followed by injection of 0.1 mL
of 5 IU of human chorionic gonadotropin (hCG; Sigma-Aldrich) approximately 48
hours later. Then the mice were immediately paired with an individual male mouse
(12-week-old). The following morning the female mice were inspected, and those
with a confirmed vaginal plug were considered fertilized. Eighteen-twenty hours
after hCG injection, the female mice with a confirmed vaginal plug were
sacrificed and cumulus-enclosed one-cell embryos (zygotes) were retrieved from
the oviductal ampulae. Only healthy zygotes were pooled, washed three times, and
cultured in 20
A total of 18 NOA female mice and 24 POF mice were administered once daily with JBD of 15 mg/kg and 30 mg/kg of body weight for 4 weeks using syringe with oral zoned needle. The control group was treated with the same volume of normal saline. Then the mice were immediately paired with an individual male in individual cages for 2 weeks. Each of the breeder males had one or two successful pregnancies before mating. The male mice were removed after two weeks and pregnancies were monitored up to 20 days. When the pregnancy was confirmed, the pregnant mice were excised in the abdomen and the number and status of fetuses were observed.
An SPSS program (ver. 12.0) was used for statistical analysis. All comparisons
of numbers of follicles at each developmental stage, the number of zygotes
retrieved, blastocyst formation rate, and ovarian mRNA expression level were
analyzed by a one-way ANOVA and
To determine whether JBD has cell cytotoxicity according to its concentration,
TOV21G cells were treated with various concentrations of JBD (1, 10, 50, 100,
500, 1000, and 5000
 Fig. 1.
Fig. 1.Cytotoxic effects of JBD on TOV21G cells.Cellviability was estimated 24
hours after JBD treatment using an MTT assay. Data were presentedas mean
In NOA mice, weights of body, ovary, and uterus did not change significantly after JBD treatment for 4 weeks. Both before and after JBD treatment, body weight was about 30 g, ovarian weight was about 0.003 g, and uterus weight was about 0.09 g. However, in POF mice, JBD treatment significantly increased all weights of body, ovary, and uterus. In the POF control group, the weights of body, ovary, and uterus were 14.63 g, 2.67 mg, and 14.1 mg, respectively, whereas they were 16.23 g, 3.67 mg, and 16.6 mg in the 15 mg/kg JBD group, and they were 16.79 g, 3.69 mg, and 19.8 mg in the 30 mg/kg JBD group (Table 3).
| JBD | Weight (mean | |||
| (mg/kg) | body | ovary | uterus | |
| NOA mice | 0 (n |
30.28 g ( |
3.48 mg ( |
91.2 mg ( |
| 15 (n |
30.51 g ( |
3.22 mg ( |
92.9 mg ( | |
| 30 (n |
30.74 g ( |
2.97 mg ( |
94.6 mg ( | |
| P |
P |
P | ||
| POF mice | 0 (n |
14.63 g ( |
2.67 mg ( |
14.1 mg ( |
| 15 (n |
16.36 g ( |
3.67 mg ( |
16.6 mg ( | |
| 30 (n |
16.79 g ( |
3.69 mg ( |
19.8 mg ( | |
| P |
P |
P | ||
To investigate whether JBD improves ovarian function in NOA mice, H&E staining was performed in ovaries. The histological characteristics of follicles at each development stage were shown in Fig. 2A. A significantly increased number of surviving follicles including the primordial, primary, secondary, and antral follicles were observed in JBD treatment of 15 mg/kg and 30 mg/kg compared to the control group (Fig. 2B). The numbers of the primordial, primary, secondary and antral/mature follicles were 67, 47, 19 and 13 in 15 mg/kg JBD, respectively, and 87, 51, 29 and 11 in 30 mg/kg JBD, which were significantly increased about two times compared to the control group (34, 29, 15, and 5, respectively). In contrast, the number of atretic follicles was decreased by about half in JBD treatment compared with the control group. The ratio of primordial to primary follicle was significantly increased by JBD treatment in a dose-dependent manner (Fig. 2C).
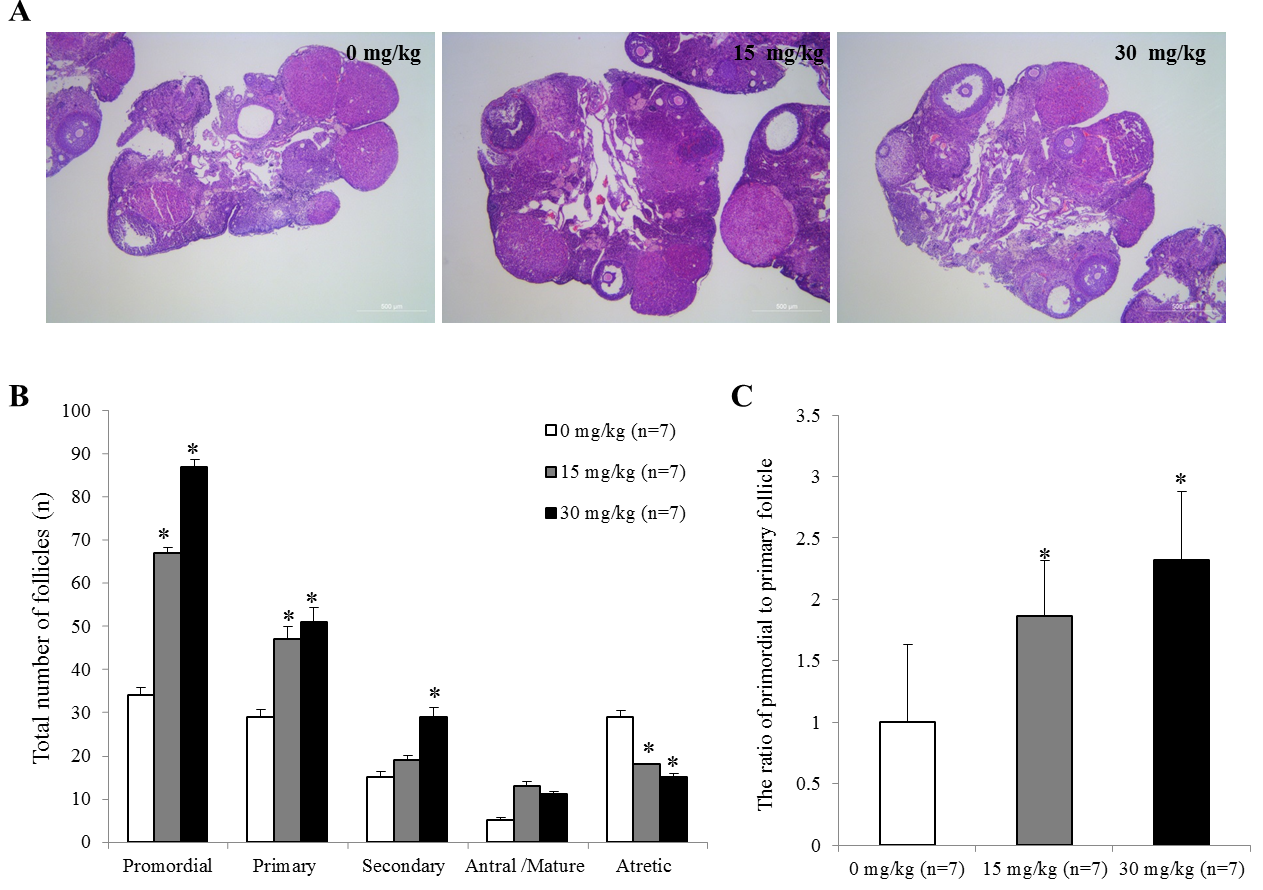 Fig. 2.
Fig. 2.Effects of JBD on follicular development. (A) The representative
histological characteristics of follicles at each development stage. (B) The
distribution of follicles at different stages. (C) The ratio of primordial to
primary follicles. Data are plotted as
mean
In order to examine whether JBD improves ovarian response and oocyte quality,
both NOA and POF mice were superovulated and mated with males after treatment of
JBD. And then, zygotes were retrieved and cultured to the blastocyst stage. In
NOA mice, the mean numbers of zygotes retrieved per mouse and embryo development
rate to blastocyst were 14.0 and 37.8% in the JBD 15 mg/kg group and 16.3 and
41.4% in the JBD 30 mg/kg group which was significantly increased compared to
6.0 and 8.8% in the control group (P
| JBD (mg/kg) | No. of zygotes | No. of zygotes | No. of | |||
| retrieved (mean |
fragmented (mean |
cultured | 2-cell embryos (%) | blastocysts (%) | ||
| 0 (con) | 42 (6.0 |
8 (1.1 |
34 | 7 (23.5) | 3 (8.8) | |
| NOA mice | 15 | 98 (14.0 |
24 (3.4 |
74 | 45 (60.8) |
28 (37.8) |
| 30 | 114 (16.3 |
15 (2.1 |
99 | 58 (58.6) |
41 (41.4) | |
| 0 (+con) |
175 (25.0 |
30 (4.2 |
145 | 122 (84.1) |
100 (68.9) | |
| POF mice | 0 (POF) | 68 (9.7 |
23 (3.2 |
45 | 8 (17.8) | 0 (0) |
| 15 (POF) | 98 (14.0 |
8 (1.1 |
90 | 74 (82.2) |
62 (68.9) | |
| 30 (POF) | 143 (20.4 |
17 (2.4 |
126 | 102 (80.9) |
83 (65.9) | |
| N | ||||||
In NOA mice, of the 6 mice in each group, the number of pregnant mice was 4
(66.7%) in the control group, 5 (83.3%) in the 15 mg/kg group, and 5 (83.3%)
in the 30 mg/kg group. There was no significant difference among groups. However,
mean number of live birthed pups was 2.83 and 3.17 in the 15 mg/kg group and 30
mg/kg group, respectively, which was significantly higher than 1.50 of the
control group (P
| Group | JBD (mg/kg) | n | Number of pregnant mice (n) | Pregnancy rate (%) | Number of live birthed pups (n) | Mean live birthed pups ( |
| 0 (con) | 6 | 4 | 66.7 | 9 | 1.5 | |
| NOA mice | 15 | 6 | 5 | 83.3 | 17 |
2.83 |
| 30 | 6 | 5 | 83.3 | 19 |
3.17 | |
| 0 (+con) | 6 | 6 | 100.0 |
46 |
7.67 | |
| POF mice | 0 (POF) | 6 | 3 | 50.0 | 14 | 2.33 |
| 15 (POF) | 6 | 4 | 66.7 | 31 |
5.17 | |
| 30 (POF) | 6 | 6 | 100.0 |
45 |
7.50 | |
To understand whether the effect of JBD relates with the activation of primordial follicle and ovarian stem cells, mRNA expressions of downstream molecules of the mTOR/PI3K and Hippo signaling pathway were examined in the ovarian tissues after the treatment of JBD. The representative downstream molecules of mTOR/PI3K signaling pathway were 4EBP1, RPS6 and S6K1, and the typical downstream molecules of Hippo signaling pathway were MST1and LATS. JBD significantly increased ovarian mRNA expressions of 4EBP1, RPS6 and S6K1in a dose-dependent manner. This effect was more remarkable at 30 mg/kg JBD than 15 mg/kg KBD (Fig. 3A). However, MST1 and LATS mRNA expressions were significantly increased just at 30 mg/kg JBD, and those expressions in 15 mg/kg KBD and control group were similar (Fig. 3B).
Oct4 is a representativepluripotent stem cell marker and ovarian mRNA expression
of Oct4 was increased in a dose-dependent manner of JBD (P
Ovarian mRNA expressions of VEGF, SDF-1
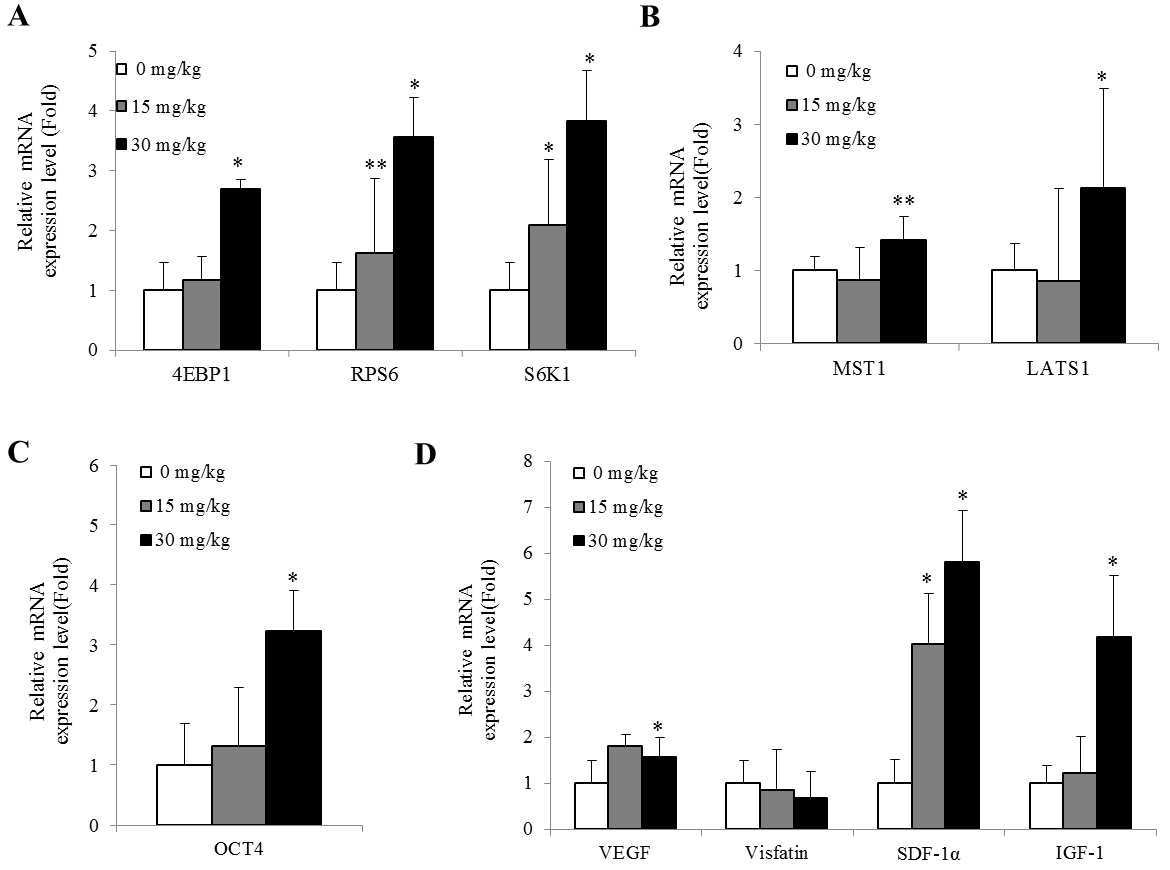 Fig. 3.
Fig. 3.Ovarian mRNA expressions of mTOR/PI3K, Hippo signaling pathway
components, ovarian stem cells marker OCT4, angiogenesis and follicular
development components inNOA mice. (A) Real time PCR analysis for mTOR/PI3K
signaling pathway components, 4EBP1, S6K1, and RPS6. (B) Real time PCR analysis
for Hippo signaling pathway components, MST1 and LATS. (C) Real time PCR analysis
forovarian stem cells marker, OCT4 and follicular development component, IGF-1.
(D) Real time PCR analysis forangiogenesis components, VEGF, visfatin, and
SDF-1
Traditional herbal medicines have been used for a long time as treatment agents for female infertility. However, the lack of modern standards for the complexity of herb ingredient and the scientific efficacy verification have made herbal medicines less widely available compared to Western medicine [37, 38, 39]. JBD also has been clinically used in Korean oriental medicine, but the exact efficacy and action mechanism are unknown. The present study showed that JBD treatment increased the number of surviving follicle from primordial follicles to antral/mature follicles in NOA mice. In addition, JBD treatment also increased numbers of retrieved zygotes, embryo development rate to blastocyst, and the pregnancy outcome including number of live birthed pups in both NOA and POF mice. This is the first study to elucidate the effect of JBD on ovarian function, ovarian response, oocyte quality, and fertility potential using NOA and POF female mice.
The number of primordial follicles decreases with female age, but dormant primordial follicles still remains in female adult around menopause and can continue to provide developing follicles and fertilizable mature oocytes for the entire reproductive lifespan [40]. Recent studies have reported that the dormant primordial follicles are activated and survived by the PI3K/mTOR signaling pathway [20, 41, 42, 43, 44, 45]. In addition, a lot of studies showed that ovarian stem cells exist in postmenopausal women ovary as wells as mouse ovary [46, 47, 48], suggesting that these ovarian stem cells can be regenerated as normal oocytes if exposed to an appropriate environment such as the young ovary [47, 49]. The activation of ovarian stem cells has been thought to be useful for recovery of ovarian function of aged ovary [49, 50]. The Hippo signaling pathway is associated with the activation of ovarian stem cells [51], and regulates ovarian function via the proliferation of ovarian stem cells [52]. The existence of ovarian stem cells in the mouse is correlated with both the age and the Hippo signaling molecules [53]. For this reason, the present study examined whether JBD may involve TOR/PI3K signaling pathway and Hippo signaling pathway, and confirmed that JBD significantly increased ovarian mRNA expressions of genes related to PI3K/mTOR (4E-BP1, S6K1, RPS6) and Hippo (MST1, LATS1) signaling pathway.
Also, ovarian angiogenesis can improve ovarian function and oocyte quality [42, 54, 55]. VEGF is a well-known representative angiogenic factor [56], and visfatin
has the angiogenic effect by itself and also significantly increases VEGF
production [57, 58]. SDF-1
In the present study, the MTT assay was performed to confirm only whether the high concentration of JBD is toxic in vitro. In vivo treatment dose of JBD was determined considering the dose actually used in clinical practice. JBD is being used locally in the treatment of female infertility such as poor responder or ovulation dysfunction in Korean traditional medicine. The daily intake dose of JBD is 1,500 mg for an average Koran women weighing 50 kg, which is about 30 mg per kg of body weight. So the present study determined two concentrations (15 mg/kg for low dose and 30 mg/kg for high dose).
The present study used two animal models for decreased ovarian function. One is natural ovarian aging (NOA) mice aged 12 months because the reproductive life span of laboratory mice including C57BL/6 strain is significantly shorter at 7-8 months [34] and C57BL/6 mice within the 11-12 month age range are near the end of their reproductive lifespan although mice of this age still become pregnant with low implantation rate and high miscarriage rate [35]. The other is POF mice that have artificially inhibited or reduced ovarian function by cisplatin. Cisplatin is a first-line chemotherapeutic agent for ovarian cancer that has been widely used to establish POF model. The mice that received 2.0 mg/kg cisplatin showed abrupt body weight loss on the 2 day and decreased locomotor activity from day 5 [63], and the numbers of primordial and preovulatory follicles in the ovary were significantly decreased at day 10 [64]. For this reason, many studies have injected 2 mg/kg cisplatin intraperitoneally daily for 10 days [65, 66]. The NOA mouse has an advantage that really reflects natural ovarian aging process. In contrast, the POF mouse take a shorter time to construct, but it is made artificially and do not really reflect natural ovarian aging process. The present study showed that JBD resulted in the increase in the production of surviving follicles, the number of oocyte ovulated, and embryo development in both mouse models.
The present study chose to recover zygotes and cultured them to the blastocyst stage for two reasons to evaluate oocyte quality. One is that embryo developmental competency is determined by oocyte quality [67]. The other is that this study aimed to know the number of oocyte ovulated as well as in vitro embryo developmental competency. The zygote can reflect the number of oocytes ovulated as fertilized oocytes. In addition, this study did not examine follicle number in POF mice because the ovarian size of POF mice induced anticancer drug is so small that no histological examination is possible. So this study exzmine the number of oocytes (zygotes) ovulated than the number of follicle in POF mice.
Improvement of oocyte quality may result to increase implatnation potential as well as embryo develoment cometency. Therefore, the present study examined the pregnancy rate and number of live birthed pups after JBD-administered NOA and POF mice with male mice to confirm whether JBD treatment increases fertility poterntial. As expected, JBD treatment significanlty increased not only pregnancy rate but also number of offspring born. This result means that JBD treatment resulted in the ovulation of more good quality oocytes.
However, one of the limitations of the present study is that this study did not reveal the critical components for the beneficial effects of JBD. JBD is a mixture of 7 kinds of herbs, which contains a wide variety of components. Catalpol is an active component of Rehmanniae radix preparata, inhibits muscle apoptosis and autophagy via the mTOR signaling pathway [68], and has antiaging activity [69]. Faavonoids, triterpenesaponins and polysaccharides are representative component of Astragali Radix and they have various biological, pharmacological and therapeutic efficacy activities includingpotent immunomodulation, antioxidant, and anti-inflammation [70]. Therefore further studies are needed to elucidate the amount of phytochemical diversities of JBD by quantitative analysis using HPLC or LC/MS.and the mechanism of action based on the main components.
In conclusion, the present study firstly reveals that JBD improves ovarian function, oocyte quality, and fertility potential in both NOA and POF mice to withstand ovarian aging. The present study also reveals that JBD results in the increased ovarian expressions of genes associated with of PI3K/TOR and Hippo signaling pathway for the activation of primordial follicles and ovarian stem cells, angiogenesis, and follicular development. These results suggest that the beneficial effects of JBD on ovarian function, ovarian response and oocyte quality may be attributed to the activation of primordial follicle, ovarian stem cells, ovarian angiogenesis and early follicular development. Therefore, these results are expected to contribute to the development of a new treatment strategy for ovarian aging or POF.
Joo JK and Lim CH designed the research study. Park MJ and Kim HJ performed the research. Kim CW and Yoon CH provided help and advice on the critical comments. Yoo JE and Joo BS wrote the manuscript as supervisors.
This study was supported by Biomedical Research Institute Grant (2018B026), Pusan National University Hospital.
The authors report no conflicts of interest.
