Perioperative antibiotic therapy is recommended to reduce the incidence of infection after caesarean section. However, the optimal timing of prophylactic antibiotic administration in such cases remains controversial. With this meta-analysis, we aimed to evaluate the safety and efficacy of prophylactic antibiotic therapy before skin incision versus after umbilical cord clamping in patients undergoing elective caesarean section. We searched the PubMed, EMBASE, Cochrane Library and Web of Science databases for randomised controlled trials (RCTs) published between January 1, 2000 and July 1, 2020. The 1101 initially identified references were narrowed to 10 RCTs involving 5020 women for the final analysis. Briefly, we determined that prophylactic antibiotic therapy before skin incision not only reduced the incidence of postpartum endometritis (relative risk (RR), 0.56; 95% confidence interval (CI), 0.34-0.92; P = 0.02), but also decreased the rate of total infectious morbidity (RR, 0.79; 95% CI, 0.64-0.98; P = 0.03) when compared to antibiotic therapy after umbilical cord clipping. However, the two timings of antibiotic administration did not lead to significant differences in the incidence of wound infection (RR, 0.73; 95% CI, 0.54-1.00; P = 0.05), maternal febrile morbidity (RR, 1.20; 95% CI, 0.67-2.14; P = 0.54), neonatal sepsis (RR = 0.65; 95% CI, 0.37-1.13; P = 0.13), septic workup (RR, 0.89; 95% CI, 0.67-1.18; P = 1.00) or neonatal intensive care unit admission (RR, 0.87; 95% CI, 0.69-1.09; P = 0.23). In conclusion, the prophylactic administration of antibiotics before a skin incision is made for an elective caesarean section can significantly decrease the incidence of total infectious morbidity and postpartum endometritis.
Caesarean section is one of the most commonly performed surgical procedures worldwide. According to recent studies, this procedure is the most common risk factor for postpartum infection, including wound infection and endomyometritis [1, 2]. Other studies have unequivocally demonstrated a 5- to 20-fold increase in the risk of maternal infection with caesarean section compared to vaginal delivery [3, 4]. These data suggest the importance of prophylactic antibiotic therapy in women undergoing caesarean section.
Recent research reports suggest that the prophylactic use of antibiotics can significantly reduce the risk of infectious diseases in women undergoing caesarean section [5, 6]. The current focus of debate in this field concerns the timing of antibiotic use during caesarean section and the maternal and foetal risks and benefits. For instance, the use of prophylactic antibiotics before skin incision might increase the risk of infection with drug-resistant organisms or could mask the incidence of neonatal infection [7, 8]. However, the use of prophylactic antibiotics after umbilical cord clamping may increase the incidence of maternal infection [8].
In recent years, research interest in the issue of prophylactic antibiotic use during caesarean section has grown rapidly. Two previous meta-analyses have reported the timing of antibiotic use during caesarean section but achieved conflicting results [9, 10]. In 2015, Zhang et al. reported no difference in the incidence of infection after elective caesarean section irrespective of whether antibiotics were administered before skin incision or after cord clamping [10]. However, Bollig et al. reported that antibiotic prophylaxis prior to skin incision reduced the incidence of infectious diseases in women undergoing elective caesarean section [9]. With this meta-analysis, we aimed to systematically assess the evidence supporting the timing of prophylactic antibiotic administration during elective caesarean section.
Two independent reviewers (HSF and WYY) conducted a literature search of the PubMed, Cochrane library, Embase and Web of Science databases to identify all relevant studies published between January 1, 2000 and July 1, 2020. Only articles published in English were identified as eligible. The following keywords were implemented in our search strategy: (‘caesarean delivery’ or ‘caesarean section’ or ‘caesarean’) and (‘antibiotics’ or ‘antimicrobials’ or ‘prophylactic antibiotics’) and (‘randomised controlled trial’ or ‘RCT’). Manual searches of the reference lists from the identified articles were also performed to screen for additional articles.
The eligibility criteria for inclusion in the meta-analysis were (1) a randomised controlled design; (2) publication; (3) elective caesarean section; (4) antibiotic prophylaxis at cord clamping vs before skin incision and (5) reporting of at least one of the following results: endomyometritis or endometritis, fever, total infectious morbidity, wound infection, sepsis workup, neonatal intensive care unit (NICU) admission (for main causes including respiratory disease during delivery and prematurity) or neonatal sepsis. Studies that were published as letters, commentaries, observational studies, review articles, conference abstracts or case reports were excluded.
Two reviewers (WYY and WYQ) used a standardised form to independently extract the following featured data from the included publications: first author, country, date of publication, number of study subjects and vital results. Two authors (HSF and WYY) independently performed a quality assessment of all the included studies according to previously published guidelines [11]. The quality assessment evaluated seven domains in each study: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias. All disagreements were resolved through discussions with another reviewer (YQ).
RevMan 5.2 software (Cochrane Collaboration) was used to perform the
meta-analysis in this study. All statistical analyses were performed according to
a fixed- or random-effects model in accordance with the level of statistical
heterogeneity across studies, which was evaluated using I
A flow chart of the study selection process for inclusion in the meta-analysis is presented in Fig. 1. Initially, 1101 studies were identified through the database search. Twenty-two studies met the eligibility criteria, and 12 were excluded after a careful reading of the full texts. Finally, 10 studies [8, 10, 12, 13, 14, 15, 16, 18, 19] including 5020 women fulfilled the criteria for inclusion in the meta-analysis. The characteristics of all included studies are listed in Table S1, and the risk of bias in each study is summarised in Table S2.
 Fig. 1.
Fig. 1.Flow chart of the literature selection process for the meta-analysis.
As shown in Fig. 2A, all 10 included studies reported wound infection [8, 10, 12, 13, 14, 15, 16, 17, 18, 19]. A fixed-effect model was adopted for this
variable because no heterogeneity was observed among the studies (P =
0.66, I
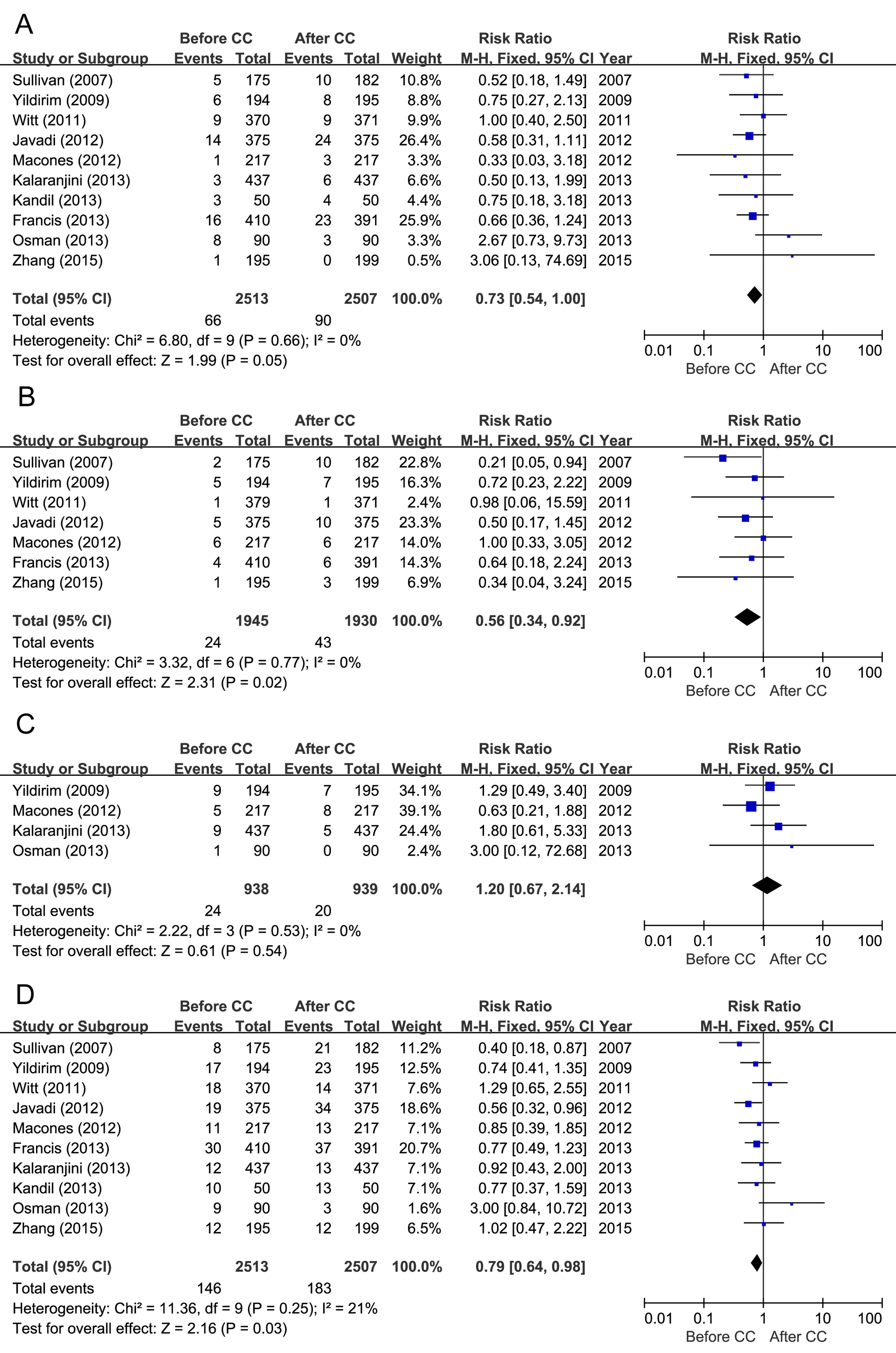 Fig. 2.
Fig. 2.Forest plots and meta-analysis of the relative risks of (A) wound infection, (B) endometritis and/or endomyometritis, (C) fever and (D) total infectious morbidity reported in the included articles.
Seven studies [8, 10, 12, 13, 17, 18, 19] evaluated the risk of endometritis
and/or endomyometritis, and no significant heterogeneity was observed among the
pooled data (I
Fever was reported in four studies [8, 14, 16, 19] no significant heterogeneity was observed among the pooled data. Therefore, a fixed-effects model analysis was used to compare the risk of fever in women who received prophylactic antibiotics before skin incision and those treated after cord clamping. This analysis yielded an RR of 1.20 (95% CI, 0.67-2.14), as shown in Fig. 2C.
All 10 studies [8, 10, 12, 13, 14, 15, 16, 17, 18, 19] reported the outcome of
total infectious morbidity, and no heterogeneity was observed among the studies
(I
The outcome of neonatal sepsis was reported in four studies [10, 14, 17, 19] and
no significant heterogeneity was detected among the studies (I
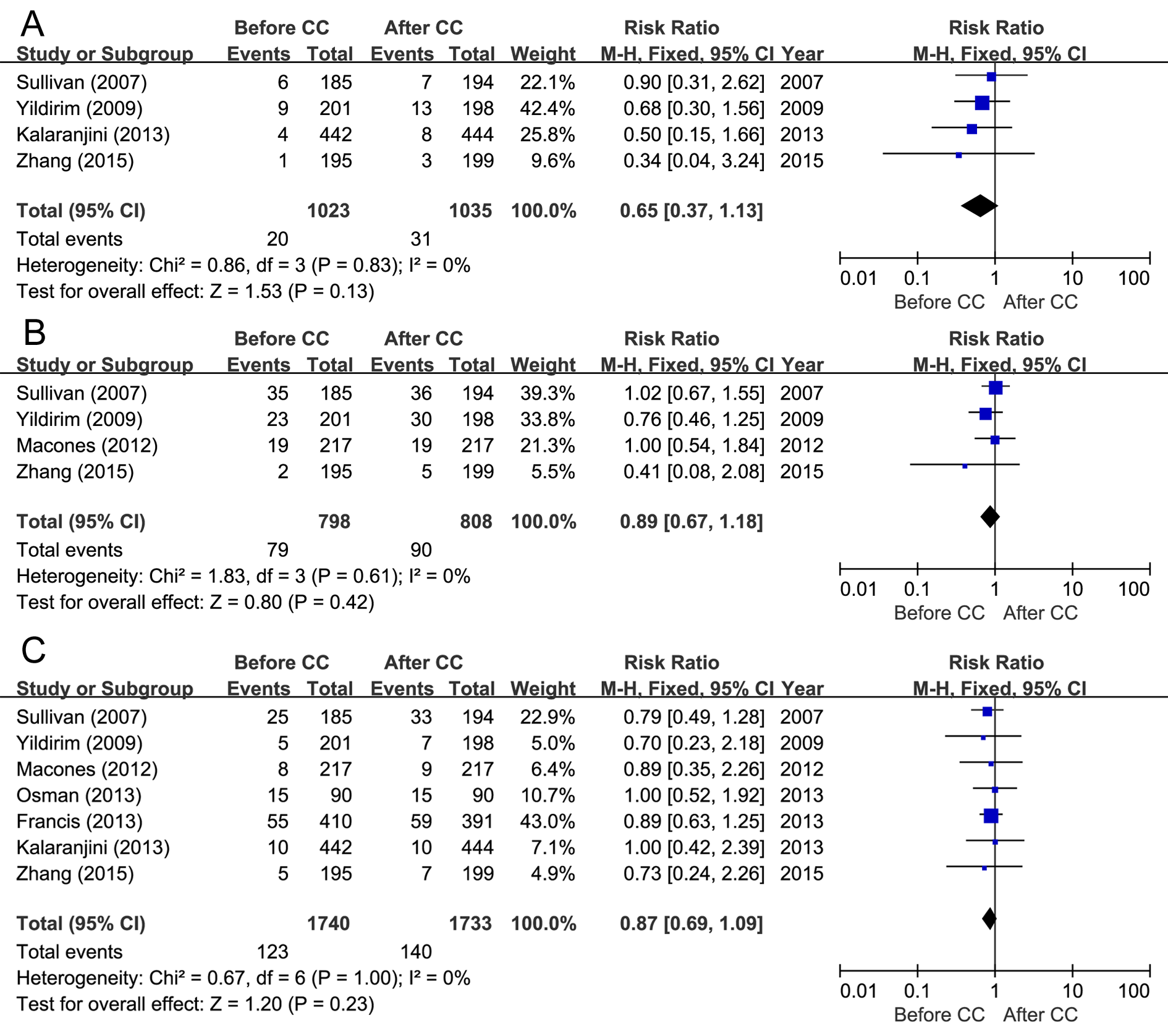 Fig. 3.
Fig. 3.Forest plots and meta-analysis of the relative risks of (A) sepsis, (B) sepsis workup and (C) neonatal intensive care unit (NICU) admission reported in the included articles.
Sepsis workup was reported in four studies [8, 10, 17, 19], and no significant
heterogeneity was detected among these studies (I
Seven studies evaluated the outcome of NICU admission [8, 10, 12, 14, 16, 17, 19], and a low level of heterogeneity was observed (I
A funnel plot was used to assess the reliability of publication bias in this meta-analysis. As shown in Fig. 4, the funnel plot for wound infection was practically symmetrical. Accordingly, there appeared to be no potential publication bias among the included studies.
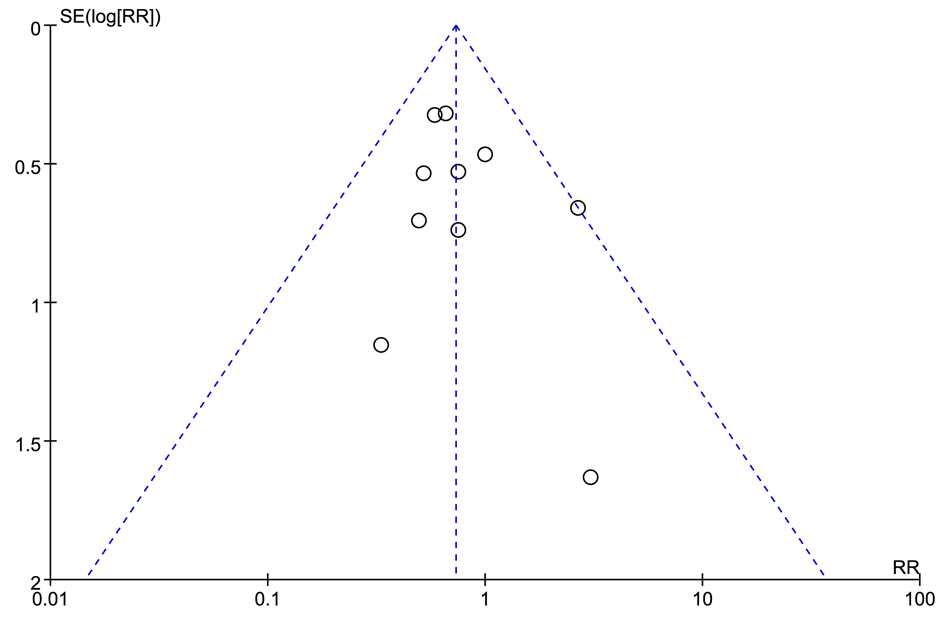 Fig. 4.
Fig. 4.Funnel plot analysis of publication bias in the included studies.
In this meta-analysis, we evaluated whether the prophylactic use of antibiotics would be more effective prior to skin incision than after umbilical cord clamping as a means of reducing the incidence of infection associated with elective caesarean section. Our evaluation of 10 studies involving more than 5000 patients revealed that the prophylactic administration of antibiotics before skin incision could significantly reduce the incidence of both endometritis (RR, 0.56; 95% CI, 0.34-0.92) and total infectious morbidities (RR, 0.79; 95% CI, 0.64-0.98) associated with elective caesarean section. However, antibiotic administration before skin incision and after cord clamping showed no significant difference in the incidence of wound infection, fever, neonatal sepsis, septic work-up or NICU admission. These findings are important because a previous meta-analysis did not observe a reduction in the risk of adverse maternal outcomes when prophylactic antibiotics were administered prior to skin incision versus after umbilical cord clamping [10].
Our results are partially consistent with those of another recently published meta-analysis [9]. However, our study was specifically designed to evaluate the optimal timing of prophylactic antibiotic administration during elective caesarean delivery. Our approach to study selection differed from that of Bollig et al. (2018) [9] in several aspects. First, we only included articles published in English. Second, we only selected articles published between January 1, 2000 and July 1, 2020. Third, we only searched the PubMed, Embase, Cochrane library and Web of Science databases for relevant literature. Therefore, the two studies analysed different sets of literature.
Prophylactic antibiotic use, which has been proven to effectively reduce the risks of postnatal wound infection and some infectious complications, has long been the standard of care during caesarean sections [5, 20]. However, the possibility that antibiotics administered before skin incision might pass through the placenta and the consequential effect of this possible event on a neonatal sepsis workup remain controversial [21]. In a previous meta-analysis that combined studies on elective or emergency caesarean section, the authors suggested that neither the prophylactic use of antibiotics before skin incision nor after cord clamping affected the neonatal outcomes [22, 23, 24]. Our results are consistent with these earlier reviews [22, 23, 24] and further confirm that the timing of prophylactic antibiotic therapy does not significantly affect the risk of adverse neonatal outcomes. However, some researchers inferred that antibiotic administration before skin incision during an elective caesarean delivery may disrupt the balance of the intestinal flora in the neonate [10]. Finally, the administration of antibiotics prior to skin incision may affect the long-term growth and development of the offspring. Future research on this topic should give more attention to the long-term growth and development of new-borns.
This study had several strengths. First, the methodology applied in this meta-analysis was rigorous because all the included studies were prospective RCTs. Second, all the included articles were rated as having a moderate or high level of quality. Third, the lack of obvious heterogeneity in the included studies indicated that our results were fairly credible and stable. Finally, the funnel plot did not reveal any effects of bias, which indicated a good research strategy.
However, several potential limitations of this meta-analysis must be considered. First, there were no unified standards for the administered antibiotic dose, timing and type across the RCTs, and this variability may have influenced the results of the meta-analysis. Second, although all included studies were RCTs, some did not describe the methods used to address allocation concealment, blinding and missing data. Consequently, there may have been a high risk of measurement bias and publication bias. Third, only studies in English were included, and therefore, relevant data from studies published in other languages may have been neglected. Finally, we included several studies with short-term follow-ups, which may have led to an underestimation of complications. Well-designed, large-scale multi-centre RCTs that apply a consistent study design and criteria are needed to obtain further evidence.
In conclusion, our results demonstrate that the prophylactic administration of antibiotics before skin incision can significantly decrease the rates of total infectious morbidity and postpartum endometritis in women undergoing caesarean section.
HSF and YQ conceived and designed the study. WYY and WYQ conducted the data searches. SFH and QY performed the analysis and wrote the manuscript. HSF revised the manuscript. YQ gave the final approval of the manuscript.
This work was supported by the National Natural Science Foundation of China (grant no. 81701509).
The authors declare no competing interests.
