Purpose of Investigation: To investigate the alterations of SCUBE1 levels in patients with missed abortion. Materials and Methods: Patients with missed abortion (n = 40) and age and gestational age-matched with normal early pregnancy cases (n = 40) were recruited for the study. All the patients’ ultrasound examinations were performed by single physician to assess the variability of conception. And to evaluate the SCUBE1 levels, venous blood samples were collected and measured with specific enzyme-linked immunosorbent assays. Results: SCUBE1 levels were found lower at missed group than control group (p < 0.001) while other hematologic parameters were similar in both groups. Conclusion: Low maternal blood levels of the SCUBE1 may be related with missed abortion by causing alterations in placental angiogenesis.
Abortion or miscarriage is defined as a clinically recognized pregnancy loss before 20th week of gestation [1, 2]. Missed abortion (MA) is one of the specific type of miscarriage that refers to a clinical abortion in which the products of conception are not expelled spontaneously from the uterus [3]. The incidence of spontaneous abortion is 8 to 20 percent while in singleton pregnancies the prevalence of missed abortion is about 2% [4, 5]. Numerous risk factors are associated with an increased risk of miscarriage, including chromosomal abnormalities, immunological factors, hereditary thrombophilias, endocrine disorders, uterine abnormalities, infections and environmental factors. Miscarriage is most commonly, caused by chromosomal abnormalities in the embryo or exposure to teratogens [6]. It is often difficult to determine the cause of a spontaneous abortion in an individual case. Angiogenesis, hematopoiesis and normal placental development are crucial for maintenance of pregnancy in early stages of embryonic life. In this context recent studies reported the relation between several gene expressions associated with angiogenesis and MA [7, 8]. A study by Fang et al. revealed the importance of HIF1α/VEGF signaling in placental angiogenesis [7]. Another study showed association between the expression of microRNA 575 (miR-575) and MA. Abnormal expression of miR-575 has been found in MA patients and it has been suggested that miR-575 influence placental angiogenesis and apoptosis [8].
Signal peptide-CUB epidermal growth factor domain containing protein (SCUBE) protein family consists of three isoforms including SCUBE1, SCUBE2 and SCUBE3. SCUBE is highly expressed as cell surface and secreted glycoprotein especially in vascular endothelium [9] and platelets [10]. Beside these cells, SCUBE expressions also have been found in many developing tissues including, central nervous system, gonads, dermomyotyome, digital mesencyheme and limb buds during the embryogenic process of mice [11].
In recent studies, it has been found that SCUBE1 is involved in primitive hematopoiesis [12] and expressed in a variety of embryonic tissues [13, 14]. Elevated serum levels of SCUBE1 have been found in acute thrombotic events such as coronary syndromes [15], ischemic strokes [16], pulmonary embolism [17] and acute mesenteric ischemia [18]. Several studies have demonstrated the relation between SCUBE levels and angiogenesis in human tissues. [19, 20].
SCUBE is highly expressed in most vascularized tissues and can be upregulated under angiogenesis related conditions in vivo, such as inflammation and hypoxia [9, 21]. SCUBE is also detectable in inflammation and hypoxia associated disease conditions [16], and its cross-talks with angiogenesis related molecules such as hedgehog (Hh) have been proven [22-25].
In light of this data, missed abortion and SCUBE1 may be associated with angiogenesis and we hypothesizd that SCUBE1 levels may show alterations in pregnant women who have experienced a missed abortion. Therefore, these findings led us to investigate the levels of a cell membrane associated protein called SCUBE in patients with MA.
This observational prospective study was conducted at Nigde Omer Halisdemir University Medical Faculty Education and Research Hospital. After obtaining ethical committee approval, all participants were received informed consent. A total of 80 pregnant women between 7 to 14 weeks’ gestation were included to this study. Forty of these pregnancies were in the missed abortion (MA) group and forty were in the gestational age matched control group. Patients with diabetes mellitus, autoimmune disease, multiple gestations, hypertensive disorders, receiving progestin derivates, history of recurrent pregnancy loss and pregnancies achieved by ART cycles were excluded from the study. Also parents with known chromosomal abnormalities were excluded from study population. MA defined as a visualized fetal pole without cardiac activity on transvaginal ultrasonography. All ultrasonographic measurements were performed by a signal experienced physician (E.D) with Mind-ray DC-7 ultrasound machine equipped with 6.5 MHz transvaginal probe (Shenzhen Mind-Ray Bio-Medical, Electronics Co, China).
Following the diagnosis MA blood samples were collected from the cannulated antecubital vein following a 30 minutes resting period. Venous blood samples were collected in 10 mL vacutainers with silica clot activator and polymer gel separator and immediately stored at 4 ℃. Next, the serum was separated from the cells and fibrin by centrifugation at 2,000 g for 15 min and stored as aliquots at -80 ℃ until assayed. Human SCUBE1 levels were measured with ELISA kit (Human SCUBE1 ELISA Kit, Sunred Biological Technology, Shanghai, China) according to instructions of the manufacturers.
Statistical analyses were performed using the SPSS for Windows 21.0 (SPSS Inc. IL, USA) software package. With the confidence interval of 95%, p < 0.05 was considered a statistically significant difference between the groups. The assumption of normality was tested via the Shapiro-Wilk test. Data are presented as means ± SD for continuous variables, median ± IQR for others. To assess the differences of variables between groups, the independent sample t test and Mann-Whitney U-test was used. Correlations between the parameters were assessed using the Spearman correlation test.
The statistical program on the website of the statistical department of the University of British Colombia was utilized to calculate the sample size and the power of our study (http://www.stat.ubc.ca/~rollin/stats/ssize/n2.html). According to this calculation, the inclusion of 35 patients in each study group with 80% confidence interval and p < 0.05 significance level was calculated as an adequate for the sample size of our study.
A total of eighty pregnant women were included in the study. The age range of patients varied between 18-40 years in the study group and 18-38 in the control group. Median gestational age was 9.2 (8-12) weeks vs. 9.2 (8-11) weeks in the study and control groups, respectively (p = 0.21).
There were no statistically significant differences in terms of maternal and gestational age, gravida and parity, abortion history, and maternal body mass index between the groups (Table 1). SCUBE1 levels were found to be lower in the missed abortion group compared to the control group (p < 0.001) (Figure 1) while other hematologic parameters were similar in both groups (Table 2). The correlation analysis in the missed abort group showed that SCUBE1 level was negatively correlated with presence of the missed abortion (r - 0.40, p < 0.0001).
| Variables Median (IQR) | Missed (n = 40) | Control (n = 40) | p |
|---|---|---|---|
| Age (year)** | 27 (12) | 26.5 ± 8.75 | 0.35 |
| Gravida** | 3 (3) | 3 (2) | 0.51 |
| Parity** | 1.5 (1.75) | 2 (1) | 0.38 |
| Abortion** | 0 (1) | 0 (1) | 0.84 |
| Gestational age(week)** | 9 (2) | 9 (2) | 0.21 |
| BMI (kg/m²)* mean ± SD | 25.24 ± 5.42 | 25.93 ± 4.56 | 0.58 |
* Independent sample t test, ** Mann-Whitney U-test.
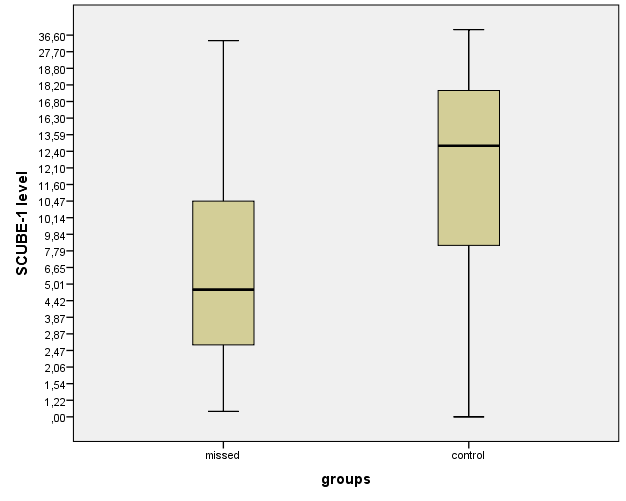 Figure 1.
Figure 1.— Distribution of SCUBE levels in the groups.
| Variables(mean ± SD) median (IQR) | Missed (n = 40) | Control (n = 40) | p |
|---|---|---|---|
| SCUBE-1 (ng/mL)** | 4.66 (7.95) | 12.7 (9.64) | 0.001 |
| WBC (K/μL)* | 9.4 ± 3.01 | 8.95 ± 2.15 | 0.45 |
| Hgb (g/dL)* | 13.3 ± 0.98 | 13.1 ± 1.01 | 0.31 |
| MCV (fL)* | 85.35 ± 5.10 | 83.3 ± 5.17 | 0.12 |
| RDW (%) ** | 13.2 (1.4) | 13.8 (1.5) | 0.21 |
| PLT (K/μL) * | 264 ± 59.5 | 257 ± 51.9 | 0.64 |
| MPV (fL)* | 9.97 ± 1.08 | 9.92 ± 1.21 | 0.86 |
| PDW (fL)** | 16 (0.62) | 16 (0.5) | 0.89 |
* Independent sample t test, ** Mann-Whitney U-test.
The result of this study demonstrated that SCUBE1 levels were lower in cases with missed abortion when compared to the control group. Although many studies have been carried out about first trimester pregnancy losses in immunological, endocrine and genetic areas, early embryonic development and maintenance of pregnancy is still unknown in many aspects [6, 20, 26-29].
Elevated SCUBE1 levels have been shown in thrombotic events such as; ischemic stroke, acute coronary syndrome, pulmonary embolism and mesenteric ischemia [30-33]. Özkan et al. showed higher SCUBE1 levels in patients with hypertension and they stated this could be a potential early marker of thrombotic events [34].
Studies mentioned in thrombotic events had a common finding of a rise in SCUBE levels. Contrary to these findings we have demonstrated a reduction in SCUBE levels. Our results are important in terms of showing that the SCUBE is not only involved in thrombotic processes, but also its potential role in the continuation of early fetal/embryonic life.
We think that this difference caused from the disparity of embryogenic life from the adulthood. Studies have been determined the role of SCUBE family in the developmental angiogenesis [20, 36-38]. Tsao et al. found that zebrafish SCUBE1 has a stimulating role in primitive hematopoiesis by acting as a BMP co-receptor to increase its signal activity during early zebrafish embryonic development and that inhibition of SCUBE1 expression resulted in a decrease in expression of genes related with primitive hematopoietic progenitors and primitive erythropoiesis [12]. Furthermore, in a study of C-F. Tu et al. revealed that inhibition of SCUBE1 gene was associated with early postnatal death due to craniofacial defects, exencephaly, loss of cranial vault and midbrain neural overgrowth [11]. Another study, by CF. Tu et al. showed that SCUBE2, which is another member of SCUBE gene family and expressed in vascular endothelium, was an important hedgehog signaling mediator during embryogenesis [10].
A possible reason for the difference in our results compared to literature may be that SCUBE1 level increases not only in thrombotic events but also when angiogenesis process continues. Since placental and uterine angiogenesis processes are intense during pregnancy, SCUBE levels will also remain high. Therefore, SCUBE1 levels may be lower in the missed abortion group compared to the live / healthy pregnancy group.
mRNA expression of SCUBE1 in abortion material could not evaluated. In addition, angiogenesis process in histological specimens of MA has not been investigated. Therefore, we could not support our results with this data which is an important limitation of our study.
In conclusion, our findings demonstrated association between missed abortion and low SCUBE1 levels. Low SCUBE levels may affect angiogenesis, which may lead to deterioration of the essential environment for the continuation of pregnancy. But larger studies are needed, espacially to determine the normal distributions of SCUBE1 level in viable pregnancy. If the exact alterations of SCUBE1 level in normal pregnancy can be determined, the decrease in the SCUBE level found in our study will be more meaningful for detecting early MA, and stillbirth etc.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Kayseri Erciyes University (approval number: 2019/625).
Thanks to all the peer reviewers and editors for their opinions and suggestions.
The authors declare no conflict of interest.
