Background: Endometriosis is a common disease in females that seriously affects quality of life. The principal pathological process of endometriosis is pelvic inflammation, and local and peripheral fibrosis. Treatment of endometriosis requires both pharmacological and surgical approaches. Vitamin C can scavenge oxygen free radicals and thus accelerate repair of damaged endometrium. This aim of this study was to investigate whether vitamin C can reduce fibrosis in endometriotic lesions. Methods: After establishing a rat model of endometriosis, vitamin C solution (vitamin C group) or physiological saline solution (control group) was injected into the abdominal cavity. We compared the indices of fibrotic endometriotic lesions between the two groups. Results: The volume of endometriotic lesions and degree of fibrosis observed in rats within the vitamin C group was significantly reduced compared with those observed in the control group. Immunohistochemistry showed that transforming growth factor-β1 (TGF-β1), connective tissue growth factor (CTGF), α-SMA, and collagen type I staining in lesions of the vitamin C group was significantly less than that observed in lesions from the control group (P < 0.05). Quantitative, real-time PCR (RT-PCR) determined that relative mRNA expression levels of TGF-β1, CTGF, α-SMA, and collagen type I in lesions obtained from the vitamin C group were significantly lower than levels measured in lesions obtained from animals in the control group. Conclusion: Vitamin C can reduce the volume of endometriotic lesions and inhibit fibrosis of lesions in rats. This study supports the use of vitamin C in the treatment of endometriosis.
Endometriosis (EM) is a disease of the endometrium (gland and interstitial tissues) where endometrial tissue is present outside of the uterus. The ovary and uterine ligament are the most common areas involved. Clinical manifestations include dysmenorrhea, pelvic pain, infertility, dyspareunia, and other symptoms [1]. Endometriosis is found in women aged 12-65 years, and its incidence rate in women of childbearing age is as high as 10% [2, 3]. 20%-50% cases of endometriosis are combined with infertility, and over 70% of patients present with pelvic pain [4].
The pathogenesis of endometriosis remains unclear. At present, Sampson’s theory of menstrual blood reflux is generally accepted [5]. This theory postulates that menstrual blood flows through the fallopian tube to the pelvic cavity during menstruation, leading to ectopic growth of endometrial cells to form endometriotic lesions. The principal pathological process of endometriosis is pelvic inflammation and local and peripheral fibrosis [6]. The scope and extent of endometriotic adhesions are specifically outlined in the revised American Fertility Society classification (r-AFS classification) of endometriosis staging where tissue width and density determine scoring. Thus, adhesions directly affect the staging of endometriosis and guide follow-up treatment. For adhesions, surgery can only separate adhesions but cannot eliminate them.
To study the mechanism that gives rise to secondary endometriosis adhesions, and investigate effective prevention and treatment strategies for these adhesions, we modeled endometriosis in rats. In this model, we implanted rat endometrium tissue ectopically under the abdominal wall. We subsequently performed intraperitoneal perfusion of vitamin C to determine whether vitamin C can treat endometriotic lesions and inhibit lesion fibrosis. The long-term goal of this study is to determine whether vitamin C can improve fertility in patients with endometriosis.
Female rats were obtained from the Experimental Animal Center of Zhejiang Province, and were raised in the animal room of the Sir Run Shaw Hospital affiliated with Zhejiang University School of Medicine. Five rats per cage were housed in a room with a 12-hour light/dark cycle and provided the same food and water. The experimental protocol used was approved by the Experimental Animal Welfare Ethics Review Committee of Zhejiang University. Female mature rats (weighing between 209 and 260 g) were randomly selected for the study and were examined for three normal estrus cycles. Animals were maintained at 24 °C ± 1 °C with 55% ± 5% humidity and fed standard rat chow.
A total of 17 rats were used to model endometriosis. Rats were anesthetized with 50% chloral hydrate (40 mg/kg) given intraperitoneally and then placed on their back. On the operating table, 10% povidone iodine was used to disinfect the lower abdomen and rats in the experimental group were operated on twice.
In the first operation, a 3-cm incision was in the midline of the abdomen, and the abdominal cavity was opened. We identified the right uterine horn (approximately 2.0 cm) and tied the mesentery with a 4-0 absorbable suture. After the blood vessels were cut off, we placed the uterine tissue into 0.9% normal saline and cut them into two 1.0 × 0.5 cm sections (Figure 1A). The endometrial surface was attached to the peritoneum and two sections were sutured to the peritoneum of the lower abdominal cavity on both sides using silk thread, as close as possible to an area rich in blood vessels. The abdomen was then closed with 4-0 absorbable suture (Figure 1B). To reduce environmental impact and tissue drying, operation times were limited to 15 minutes. All rats were housed for 2 weeks after the first operation. During this time, all rats were housed and fed as indicated above but none of them were given any medication.
 Figure 1.
Figure 1.— Construction of rat endometriosis model by endometrial transplantation. (A) Macroscopic features of transplanted endometrium. (B) Autologous endometrium transplanted on the abdominal wall of rat. (C) Endometriotic lesion formed after 2 weeks of transplantation.
After 2 weeks, three rats were randomly selected and administered a lethal dose of 50% chloral hydrate. The abdominal cavity was dissected and endometriotic lesions were clearly visible on both sides of the abdominal cavity in each of the three rats. The tissue from these rats was fixed and embedded in paraffin, serial sections cut, and Masson’s staining performed on sections to examine endometriotic lesion formation and fibrosis (Figure 2).
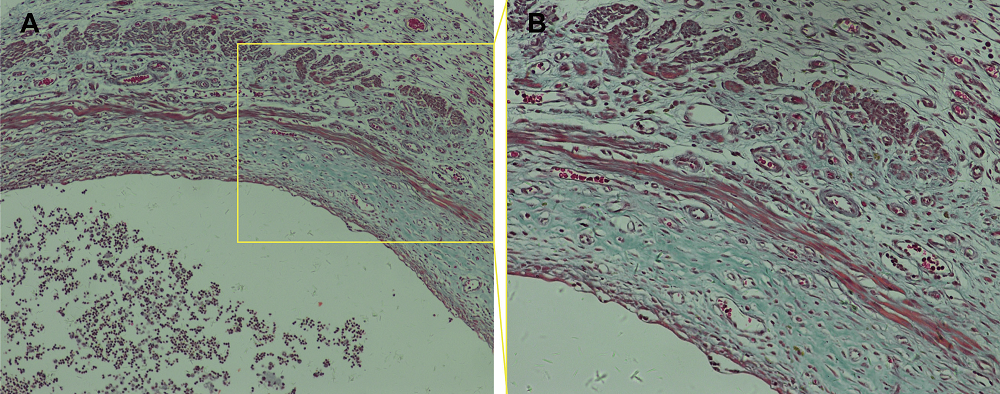 Figure 2.
Figure 2.— Representative photomicrograph of the cystic walls from rat endometriotic lesions after Masson’s staining (A: 100 ×; B: 200 ×).
The remaining 14 rats were randomly divided into two experimental groups (Table 1). Over the next 2 weeks, the vitamin C group (n = 7) was perfused intraperitoneally with a vitamin C solution (in 0.9% saline) at 500 mg/kg per day. The control group (n = 7) was perfused intraperitoneally with 0.9% physiological saline over the same 2 week period.
| Control Group (n = 7) | Vitamin C Group (n = 7) | P Value | |
|---|---|---|---|
| Body Weight (g) Before perfusion | 232.02 ± 17.44 | 231.94 ± 16.54 | > 0.05 |
| Body Weight (g) After perfusion | 233.85 ± 17.64 | 234.28 ± 16.37 | > 0.05 |
At the end of the 2 week treatment period, rats in the two experimental groups had a second laparotomy performed after a lethal dose of 50% chloral hydrate. Intra-abdominal endometriotic lesions obtained from the vitamin C group and the control group were evaluated (Table 2, Figure 3 and Figure 4). The lesion size of the abdominal wall of each rat was measured by a uniform scale of length × width × height (cm), thus resulting in 14 sets of data obtained from each group of rats. After removal of the lesion, selected portions of these tissues were randomly taken and stored in 10% formaldehyde solution for histopathological examination. The remaining tissues were preserved in liquid nitrogen.
| Control Group (n = 14) | Vitamin C Group (n = 14) | |
|---|---|---|
| Mean volume of endometriotic lesions (mm3) | 246.00 | 40.917 |
| P Value | < 0.05 | < 0.05 |
Note: There were 7 rats in each group, and the lesions on both two sides of the abdominal wall of each rat were measured. Therefore, there were 14 samples in each group.
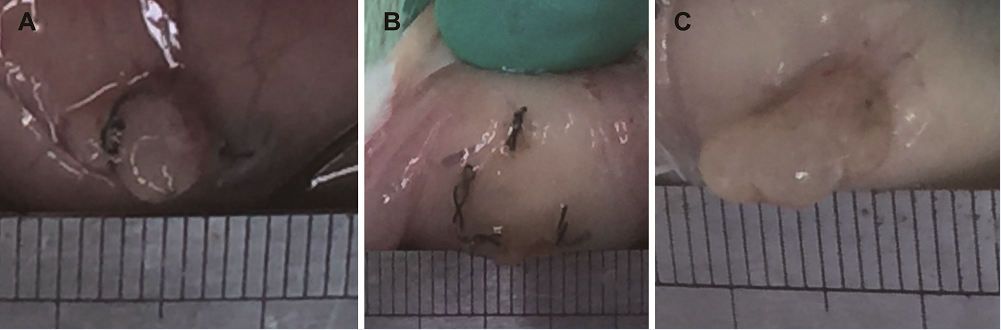 Figure 3.
Figure 3.— Size comparison of endometriotic lesions between vitamin C and control groups. (A and B) Representative endometriotic lesion of vitamin C group. (C) Representative endometriotic lesion of control group.
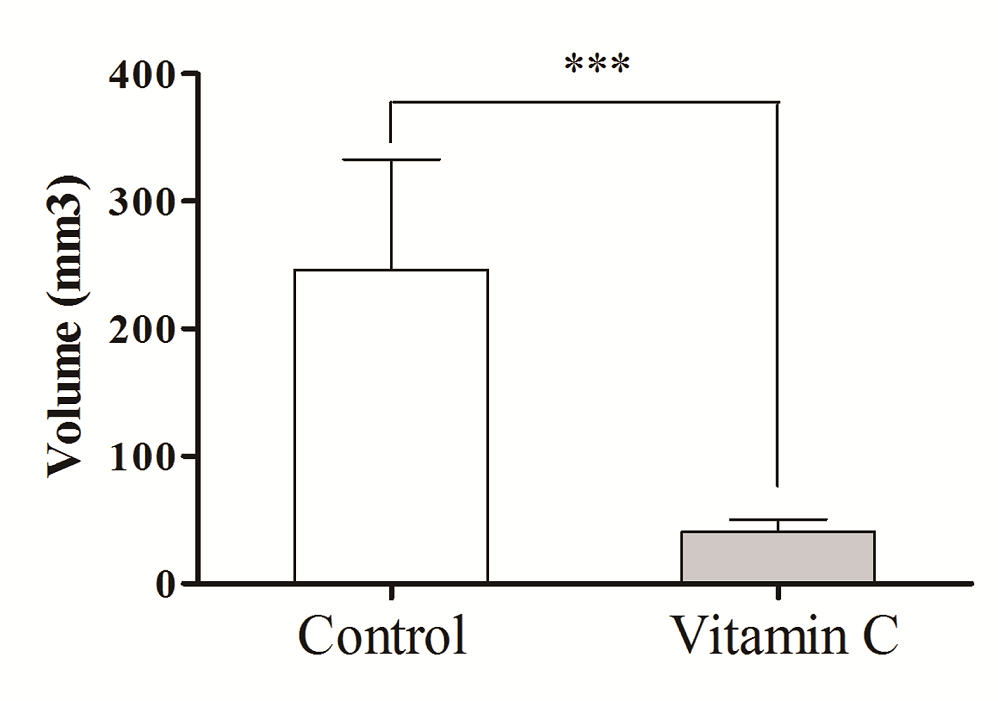 Figure 4.
Figure 4.— Comparison the size of endometriotic lesions between control group and vitamin C group.
Formation of endometriotic lesions and degree of fibrosis within endometriotic lesions were compared in tissue taken from the two experimental groups. Tissues were fixed in 10% formaldehyde solution for 24 hrs, subsequently embedded in paraffin, and serially sectioned. Tissue sections were stained with hematoxylin and Masson’s stain. After complete resection of the bilateral and subperitoneal endometriotic lesions in the vitamin C and control groups, several samples were randomly selected from each group for Masson’s staining. Under light microscopy, epithelial cells were semiquantitatively evaluated and scored as follows: 3 = 1/4 well-preserved epithelial layer; 2 = 1/4 degree preserved epithelium and leukocyte infiltration; 1 = 1/4 poorly preserved epithelium (occasional epithelial cells); 0 = no epithelial cells observed [7]. Matrix elements surrounding the tissue and the degree of fibrosis were assessed by Masson’s staining as follows: Grade 0, no fibrosis; Grade 1, minimum fibrous tissue growth; Grade 2, irregular fibrous tissue growth; Grade 3, concentric fibers and transparency [8, 9]. In the vitamin C group, four endometriotic lesions from different rats were randomly selected for analysis. In the control group, five endometriotic lesions were randomly selected from different rats. Three pathologists examined the nine lesions (Table 3) in a blinded fashion. The remaining lesions of both groups were used for quantitative, real-time PCR (RT-PCR).
| Control Group (n = 5) | Vitamin C Group (n = 4) | |
|---|---|---|
| Fibrosis score | 2.40 | 1.83 |
| P Value | < 0.05 | |
Immunohistochemical staining was used to detect endometriosis and the severity of fibrosis around endometriotic lesions. After the bilateral and subperitoneal endometriotic lesions were completely removed from rats in both the vitamin C and control groups, four samples were selected from the vitamin C group and five samples from the control group for immunohistochemical analysis. Tissues were fixed in 4% paraformaldehyde and after fixation, tissues were deparaffinized in xylene, rehydrated through a series of ethanol washes, and finally rinsed in PBS. Endogenous peroxidase activity was blocked by incubating the sections in 0.3% H2O2 for 10 minutes at room temperature. The sections were then blocked in PBS containing 5% normal goat serum (Yeasen Biotech, Shanghai, PRC) for 20 min.
Primary antibodies specific for transforming growth factor-β1 (TGF-β1), connective tissue growth factor (CTGF), alpha smooth muscle actin (α-SMA), and collagen type I were used (1 : 200; Beyotime, Haimen, PRC). Paraffin sections were incubated in primary antibody overnight and Horseradish Peroxidase (HRP)-conjugated goat anti-rabbit IgG (1 : 50; Beijing Zhongshan Biotechnology Co., Ltd.) secondary antibody was incubated for 30 minutes. Immunohistochemistry was developed using diaminobenzidine (DAB), and sections subsequently counterstained with hematoxylin (sections used for image analysis were not counterstained). Phosphate-buffered saline (PBS) was substituted for primary antibody as a negative control. Immunohistochemical staining results were automatically examined using a Leica Qwin computer image analysis system (Leica, Germany). Under low power (i.e., 200 ×), the over-stained area at edges of section were avoided and 10 fields of view were randomly chosen for analysis.
Collagen type I, α-SMA, CTGF, and TGF-β1 mRNA were quantified in endometriotic lesions and ovarian tissue by RT-PCR. Total RNA was extracted from tissues using Trizol reagent (Invitrogen, USA). Synthesis of cDNA was performed in 25 μL reaction volumes using reverse transcriptase and random primers (Promega, USA) according to the manufacturer’s instructions. RT-PCR was conducted using Bestar™ RT-PCR Master Mix (DBI Bioscience, Germany). Thermocycling consisted of an initial denaturation step of 30 seconds at 95 °C, followed by incubation at 95 °C for 15 sec, annealing at 60 °C for 30 sec, and extension at 72 °C for 30 sec. The total amount of fluorescent product was measured with a single data acquisition at the end of each cycle. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control. The relative expression level of the target gene was calculated using the 2-ΔΔCt protocol.
All data were analyzed using SPSS 21.0 statistical software. Measurement data are expressed as mean ± standard deviation. Comparison between the two groups was performed using the independent sample t-test and ratios between the two groups were compared using the χ2 test. P < 0.05 was considered statistically significant.
We measured no significant difference in body weight between rats in the vitamin C group and the control group (Table 1). However, intra-abdominal lesions in the abdominal wall of rats were reduced after intraperitoneal infusion of vitamin C solution compared with controls. In the vitamin C group, no abnormal endometriotic lesions were found in the abdominal wall of one of the rats following the second operation. There were also no observable endometriotic lesions on one side of the abdominal wall in another vitamin C-treated rat. Tabulated lesion volume of the vitamin C and control groups of is shown in Table 2, as well as Figures 3 and 4.
In tissue sections, the degree of fibrosis as highlighted by Masson’s staining of endometriotic lesions was significantly less in the vitamin C group than in the control group (P < 0.05) (Figure 5). Three pathologists blindly examined all tissue sections, and the fibrosis score of the vitamin C group was significantly less than that in the control group (Table 3). Additionally, collagen type I, α-SMA, CTGF, and TGF-β1 expression in immunohistochemical staining was significantly lower in the vitamin C group than in the control group (Figure 6). Moreover, the degree of fibrosis around endometriotic lesions in rats was significantly reduced in the vitamin C group compared with the control group.
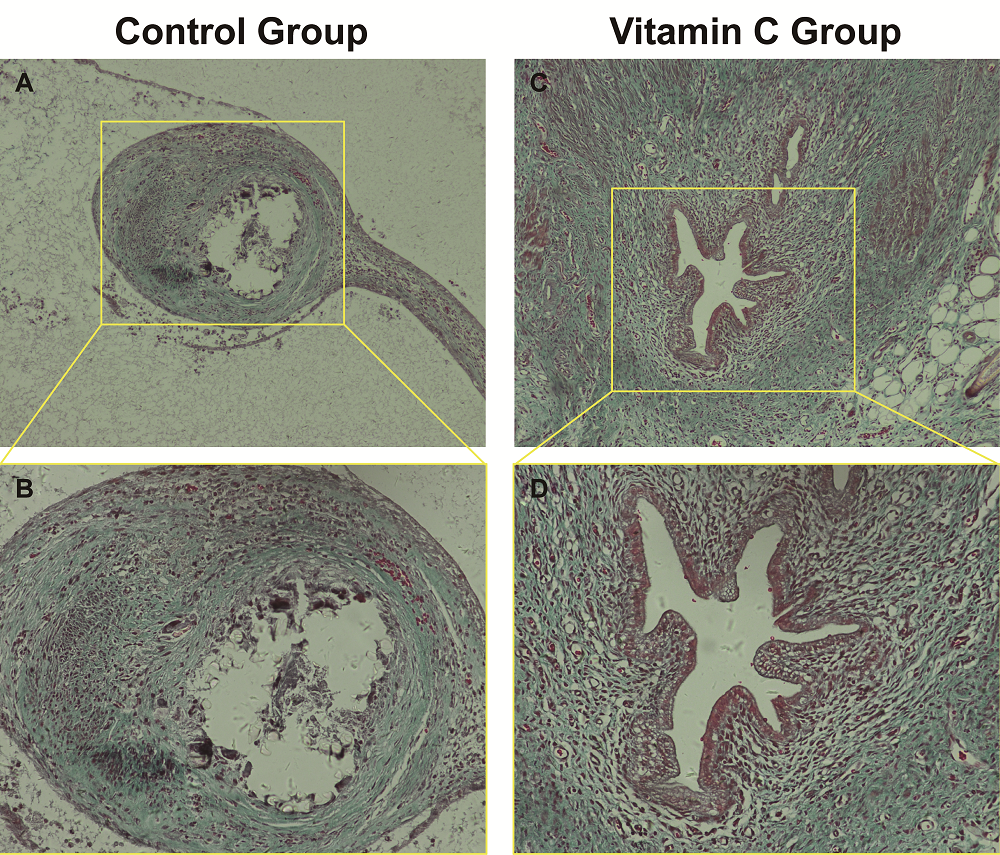 Figure 5.
Figure 5.— Comparison of the degree of fibrosis between the two experimental groups by Masson’s staining. (A and B) and (C and D) represent Masson’s staining of endometriotic lesions in control group and vitamin C group respectively. (A, C: 100 ×; B, D: 200 ×).
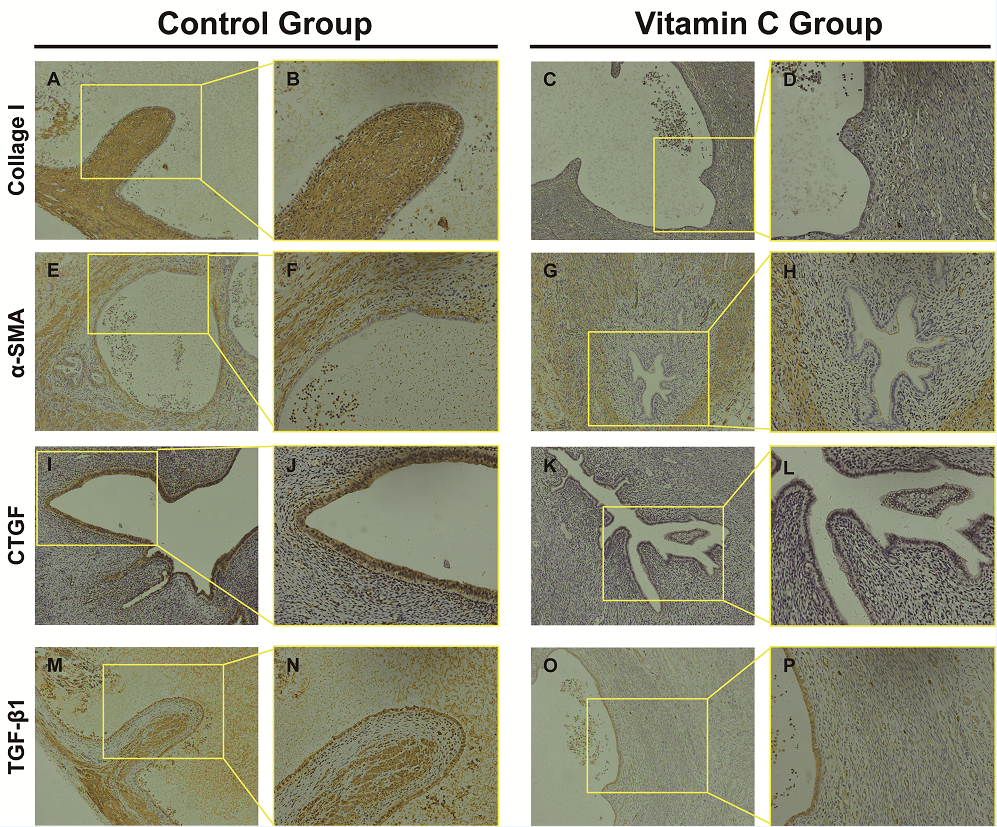 Figure 6.
Figure 6.— Comparison the expression of Collagen I, α-SMA, CTGF and TGF-β1 between control and vitamin C groups by immuniohistochemistry. (A and B) Collagen I staining of control group; (C and D) Collagen I staining of Vitamin C group. (E and F) and (G and H) represent α-SMA staining of control group and vitamin C group respectively. (I and J) and (K and L) demonstrate CTGF staining of control group and vitamin C group, respectively. (M and N) and (O and P) represent TGF-β1 staining of control group and vitamin C group, respectively. (A,C,E,G,I,K,M,O: 100×; B,D,F,H,J,L,N,P: 200×).
Quantitative RT-PCR analysis showed that TGF-β1, CTGF, α-SMA, and collagen type I mRNA levels in the vitamin C group were significantly lower than those in the control group (Figure 7). These findings are consistent with results obtained in our immunohistochemistry studies.
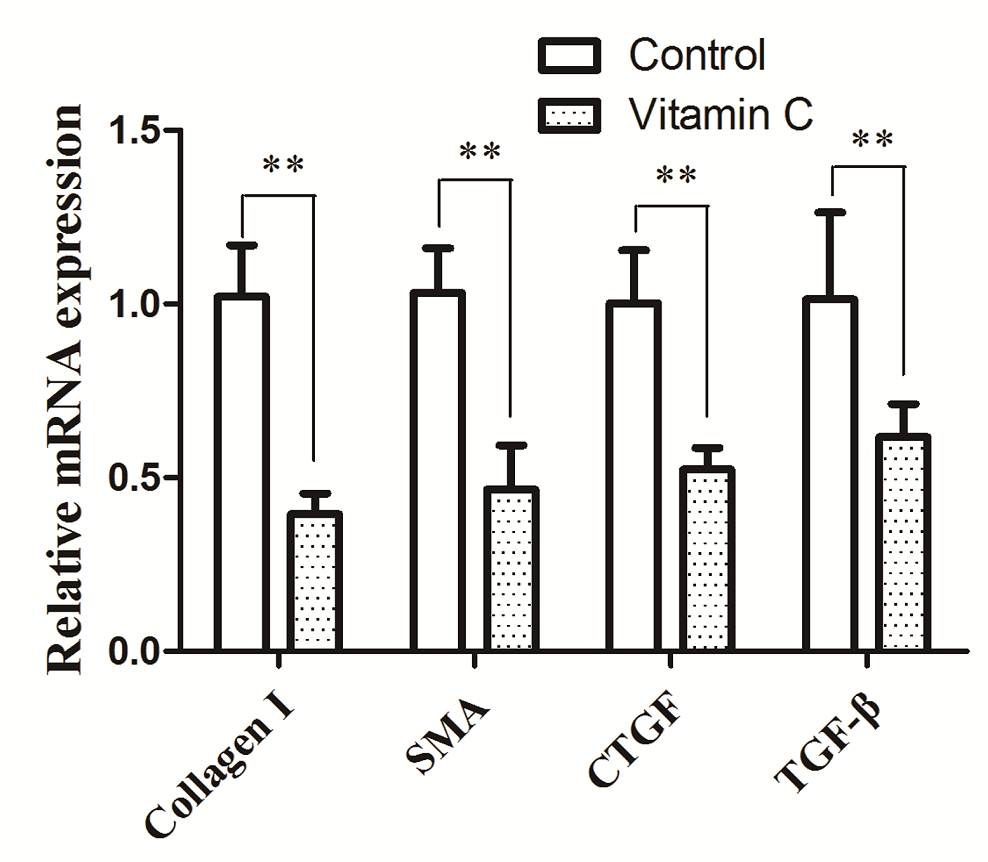 Figure 7.
Figure 7.— Relative mRNA expression levels of the fibrotic markers Collagen I, α-SMA, CTGF, TGF-b1 in endometriotic lesions from control and vitamin C group.
Endometriosis seriously affects a woman’s quality of life. Laparoscopy is recognized as the gold standard for diagnosis and treatment of endometriosis [10]; however, the recurrence rate of patients with endometriosis within five years after laparoscopic surgery is as high as 40%-50% [11]. For patients with severe deeply infiltrating endometriosis (DIE), thorough surgical procedures may lead to corresponding surgical complications such as ureter, bladder, and intestinal damage, as well as the potential risk of denervation of the pelvic floor [12]. Therefore, patients with mild clinical symptoms, fertility requirements, and postoperative patients need to take medications [13].
The specific mechanism responsible for pathological damage to surrounding tissues in endometriosis is unclear. The ovarian endometrial ectopic cyst wall is surrounded by fibrous tissue containing hemosiderin-rich macrophages and endometrial stromal epithelial cells [14]. Furthermore, in endometriosis, the number of antral follicles in ovarian tissue surrounding the cyst is reduced [15]. This phenomenon occurs in ovaries even when the endometriotic cysts are less than 4.0 cm in diameter [16]. Compared with other non-hormone-dependent tumors such as teratomas, endometriotic cysts only cause mechanical expansion of ovarian tissue and do not significantly affect ovarian reserve function [17]. Studies have also shown that laparoscopic ovarian endometriosis cystectomy damages the ovaries by removing surrounding ovarian tissue along with the cyst due to severe adhesion between the cyst and ovary [18] and that this can directly negatively affect fertility. Therefore, we propose that fibrosis associated with the endometriotic cyst is an important event that results in pathological damage, and that the degree of fibrotic adhesion determines severity of the disease. For patients with ovarian endometriosis, determining how to remove the lesion and reduce the degree of fibrosis around the endometriotic lesion is essential.
The principal feature of fibrosis is the excessive proliferation of fibroblasts and deposition of extracellular matrix (ECM). In fibrosis, homeostasis between synthesis and degradation of tissue components of the ECM is disrupted, and excess ECM replaces parenchymal tissue. Consequently, organizational structure and function are destroyed. In this study, we established an endometriosis model in the abdominal wall of rats. In this model, endometrial lesions were observed by laparotomy, and confirmed as endometriotic cysts by pathology, and fibrotic components were observed using Masson’s staining.
Foods that are rich in vitamin C can reduce the risk of endometriosis [19]. Vitamin C is a water-soluble vitamin, which functions as an antioxidant by directly scavenging oxygen free radicals. Because vitamin C can scavenge oxygen free radicals it can accelerate repair of damaged endometrium [20], and effectively inhibit endometriosis [21]. In endometriosis, reducing the lesion while limiting the degree of fibrosis around the lesion is clinically important. In our study, we administered vitamin C solution and physiological saline intraperitoneally, and compared the size of endometriotic lesions after perfusion. Lesions in the vitamin C group were significantly reduced compared with those in the control group (Table 2, Figures 3-4), and even the original endometriotic lesions in three of the rats given vitamin C disappeared. Our findings suggest that vitamin C has a therapeutic effect on endometriosis, and could also be useful in treatment of peripheral fibrosis.
The TGF-β1 signaling pathway plays an important role in the process of organ fibrosis [22, 23]. This pathway not only stimulates the growth of mesenchymal cells, but also causes an increase in collagen and fibronectin accumulation within the ECM and inhibits degradation of the ECM. This has an important initial effect of promoting formation of fibrosis [24] and, not surprisingly, TGF-β1 is significantly associated with fibrosis of endometriotic lesions [18]. High expression of TGF-β1 in endometriotic lesions leads to proliferation of fibroblasts, collagen deposition, and fibrin formation and this ultimately leads to the occurrence of pelvic fibrosis and extensive pelvic adhesions [25, 26].
CTGF is a cysteine-rich cytokine with multiple biological functions. CTGF has similar biological functions as TGF-β1 and is a downstream response element of TGF-β1 signaling [27-30]. TGF-β1 triggers monocyte chemotaxis of neutrophils and lymphocytes, enhancing the ability of these cells to secrete CTGF. TGF-β1 stimulates secretion of CTGF through a paracrine mechanism involving interstitial fibroblasts, and, in turn, CTGF stimulates the secretion of TGF-β1 [31]. CTGF can promote cellular proliferation, increase the expression of cell adhesion molecules, and promote collagen synthesis. Overexpression of CTGF leads to fibrosis and, conversely, inhibition of CTGF can inhibit fibrosis, which is beneficial to normal tissue repair and restoration of normal structural function. Therefore, studying TGF-β1, CTGF, collagen type I, and α-SMA can indirectly lead to understanding of the degree of fibrosis around endometriotic lesions.
Vitamin C contributes to the immune defense system and supports the innate and adaptive immune system of various cellular functions. For example, vitamin C can be reduce TGF-β1 levels in tissues, thereby improving fibrosis [32]. In our model of endometriosis, we found that the degree of fibrosis in the vitamin C group was significantly less than that in the control group. Specifically, immunohistochemistry showed that collagen type I, α-SMA, CTGF, and TGF-β1 expression in the vitamin C group was lower than that observed in the control group. Moreover, these findings are supported by RT-PCR assays which measured lower levels of each transcript in vitamin C treated animals.
In summary, our findings support that vitamin C plays a protective or therapeutic role in endometriosis in rats. In humans, whether intake of vitamin C in the diet of women of childbearing age can prevent or treat endometriosis remains to be determined.
The authors thank Ellen Knapp, PhD, for editing the English text of a draft of this manuscript.
This work was supported by grants from the National Natural Science Foundation of China grant 81671435 and Key Research and Development Program of Zhejiang Province grant 2017C03022.
The authors declare no competing interests.
