Primary ovarian leiomyoma is a rare type of benign neoplasm. Ovarian leiomyoma cases until a recent date usually showed favorable pregnancy outcomes. Contrary to others, the present authors report a case of a nine-week miscarriage with a giant primary ovarian leiomyoma. This neoplasm originated from the ovary with estrogen secreted by the endocrine organs. Hormone secretion is increased during pregnancy, particularly in the first trimester; it is supposed that it stimulated growth and progression of the mass. A close examination in adnexa is necessary at prenatal check.
The most common site of leiomyoma is the uterus. It develops in any location where smooth muscle cells are found. Leiomyoma that originates from the ovary is a rare case, accounting for 0.5-1% of benign ovarian neoplasms [1]. Primary ovarian leiomyoma usually occurs in perimenopausal women, often unilaterally, and coexists with uterine leiomyoma [2].
Since ovarian leiomyoma is frequently asymptomatic and very small, it is diagnosed incidentally during surgery or gynecologic exams. As size of the tumor increases, symptomatic signs, such as abdominal pain, palpation of mass, ascites, hydronephrosis, and Meig’s syndrome are more likely to appear. Unusual locations and protean imaging manifestation make differential diagnosis more challenging; histopathological analysis is usually required to confirm the diagnosis.
About 80 cases of ovarian leiomyoma have been reported in the literature to date [1], and a few ovarian leiomyomas during pregnancy have been reported. Most ovarian leiomyomas detected during the second trimester of gestation, had favorable outcomes of pregnancy [3-10].
Herein, the authors report a woman who had a miscarriage at nine weeks with a giant primary ovarian leiomyoma.
A 34-year-old nulliparous woman at nine weeks of intrauterine pregnancy presented acute abdominal pain with vaginal bleeding and palpable mass in the abdominal cavity. There were unremarkable findings on previous prenatal check four weeks prior. The pain was located diffusely in the abdomen, and her vital signs were stable. On physical examination, the measured height of the uterine fundus was at 20 weeks. Ultrasonography scanning showed a giant homogeneous hypoechoic mass in the abdominal cavity and a nine-week-sized embryo by crown-rump length, without heartbeat. The laboratory test results, including the complete blood count and levels of tumor marker, such as CA125 and CA-19-9, were within normal limits. Abdominal pelvic CT revealed that the uterus was deviated to the right, with a well-margined, 17-cm-sized mass, with a uniformly solid consistency, was located in the pelvic cavity surrounded by the left salpinx. There were no suspicious malignancy findings, such as ascites, metastasis, or enlarged lymph nodes (Figures 1A-B).
 Figure 1.
Figure 1.— CT image and gross feature of primary ovarian leiomyoma. A) Transverse imaging: the uterus is deviated to the right; a large well-margined mass has a uniformly solid consistency in the pelvic cavity surrounded by the left salpinx. B) Sagittal imaging: there are no suspicious malignancy findings, such as ascites, metastasis, or enlarged lymph nodes. C) The uterus is displaced toward the right and forward. There are no remarkable findings in the right ovary. It has a round shape and well-circumscribed margin within the left ovary capsule without grossly normal ovarian tissues and is distinct from the uterus without coexistent leiomyoma. D) Left oophorectomy specimen: 17×12.5-cm-sized white solid mass.
Under general anesthesia, an emergency exploratory laparotomy was performed via a Pfannenstiel incision. A 17×12.5-cm solid mass with a round shape and well-circumscribed margin within the left ovarian capsule without grossly normal ovarian tissues was found. It was distinct from the uterus without coexistent leiomyoma. The uterus was displaced toward the right and forward (Figure 1C). There were no remarkable findings on the right ovary, appendix, bowel, liver, and gallbladder. After performing the left oophorectomy, the authors found a white tumor with smooth external surface, showing whorled appearance on the cut section (Figure 1D). The patient was discharged without any postoperative complication. She had spontaneous and regular menses without any complaint.
| Author | Maternal |
GA1 at |
GA at |
Size of mass (cm) |
Increasing |
Pregnancy |
|---|---|---|---|---|---|---|
| Olshausen (1907) [9] | 38 | - | 12 | Man’s head size | Abrupt growing3 | Term birth |
| Moore and Forks (1945) [8] | 34 | - | 12 | 16×13 | Abrupt growing | Term birth |
| Daniel et al (1997) [4] | 31 | Term | Term | Right: 8×5, |
Incidental diagnosis4 | Term birth |
| Kohno et al. (1999) [7] | 30 | 16 | 20 | 23×23×20 | Abrupt growing | Term birth |
| Hsiao et al. (2007) [5] | 42 | Before conception | Term | 4.5×4.4×32 | 28.6% | Term birth |
| Zhao et al. (2014) [10] | 28 | Before conception | 14 | 18×16×10 | 650% | Term birth |
| Kim (2016) [6] | 35 | Before conception | 10 | 9.3×7.8 | 28.6% | Term birth |
| Abdessayed et al. (2017) [3] | 32 | - | 18 | 6.0×5.5 | Incidental diagnosis | Miscarriage |
| Current case | 34 | - | 9 | 17×12.5 | Abrupt growing | Miscarriage |
1 GA : gestational age. 2 Increasing ratio of mass: maximum (diameter of mass at diagnosis diameter of mass at surgery)/diameter of mass at diagnosis. 3 Abrupt growing: non-specific lesion in both adnexa at prenatal check. Diagnosed by symptom depending on rapid growth. 4 Incidental diagnosis: non-specific symptom of tumor. Incidental diagnosis by surgery or regular prenatal exam.
On microscopic examination, the tumor was similar to uterine leiomyoma and consisted of bland spindle cells with no mitotic activity. Immunohistochemical stain results were positive for smooth muscle actin in tumor cells (Figure 2).
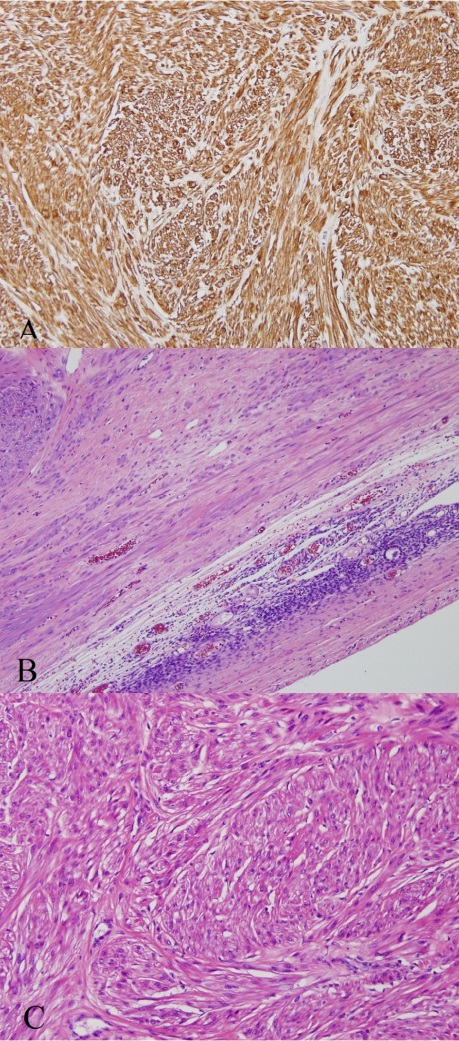 Figure 2.
Figure 2.— Microscopic finding of primary ovarian leiomyoma A) The tumor is well circumscribed. The ovarian stroma is visible on the right lower portion (Hematoxylin & Eosin staining, ×100). B) The tumor consists of spindle cells arranged in inter- secting fascicles (Hematoxylin & Eosin staining, ×200). C) The tumor cells are diffusely positive for smooth muscle actin (immunohistochemical staining, ×200).
Estrogen was suggested as a potential stimulator for tumor development [11]. One of the theories suggest that a smooth muscle cell in the ovarian hilar blood vessel may be a possible origin [12]. Other theories suggest the following as potential origins: Ovarian ligament, smooth muscle cells, and multipotent cells in the ovarian stroma, undifferentiated germ cells or cortical smooth muscle metaplasia of endometriosis stroma, smooth muscle present in mature cystic teratoma and smooth muscle in the wall of mucinous cystic tumor [2, 13]. Several theories have discussed the origin of primary ovarian leiomyomas. However, this topic remains controversial.
Considering its rarity, ambiguous location, and characteristics of the primary ovarian leiomyoma, physicians can make differential diagnoses among ovarian fibroma, thecoma, broad ligament leiomyoma, and pedunculated leiomyoma [14]. The potentially useful modalities to detect extrauterine leiomyomas and determine whether these are malignant or benign tumors include ultrasonography, CT, and MRI. A low-signal intensity similar to that of the smooth muscles on T2-weighted images is particularly valuable in characterizing these tumors [15]. The present authors initially performed an ultrasonographic examination and confirming a definite feature of leiomyoma, CT was performed subsequently for differential diagnosis. Ovarian leiomyoma has grossly variable characteristics, some are solid and others may be accompanied with calcification, hyalinization, hemorrhage, and necrosis. On microscopic evaluation smooth muscle cell spindles were observed. Immunohistochemical staining, which is specific to the smooth muscle cells, is useful in differential diagnosis.
The prevalence of uterine leiomyomas detected by research-quality ultrasound screening in the first trimester of pregnancy accounts for 10.7% [16]. During pregnancy, the blood flow in the uterus, estrogen and progesterone levels, and possibly human chorionic gonadotropin levels increase. Uterine leiomyoma may occur in response to hormones and may regress after pregnancy [4]. The tumor size is main-tained during gestation in 50-60%, increased in 22-32%, and decreased in 8-27%. If it increases in size, most of the growth occurs in the first trimester. During the pregnancy, the mean ratio of increasing volume is 12%, and more than 25% is unusual [17, 18]. This case was diagnosed as a giant ovarian leiomyoma in pregnancy, and the possibility of tumor growth during the first trimester was speculated.
Most pregnant women do not develop complications related to uterine leiomyoma. In those with leiomyoma-related complications, degeneration change is the most common occurrence. Malpresentation, preterm labor and delivery, miscarriage, and placental abruption also appeared to increase the risks [19]. Most of ovarian leiomyomas during pregnancy reported to date, usually showed favorable pregnancy outcomes. Although miscarriage is a common complication in first trimester, there were no clinical and ultrasound findings to indicate factors of miscarriage. Several hypotheses on the mechanisms that could adversely affect pregnancy stability are proposed. The potential hypothesis on spontaneous abortion in this case is that the rapid growth of the leiomyoma may have increased the uterine contractility or have altered the placental catalytic enzyme production [20].
The present authors emphasize the importance of definite adnexal evaluations in early pregnancy. If solid masses arising from the ovaries are found, ovarian leiomyoma should be suspected. When a suspicious ovarian leiomyoma is detected, serial examinations are recommended, particularly during the first trimester.
