Poor maternal vitamin D status is associated with adverse pregnancy outcomes such as preeclampsia, gestational diabetes. And increased risk of caesarean delivery. The authors conducted a prospective observational study to determine the association between maternal serum vitamin D levels and spontaneous preterm delivery. Pregnant women between 26 and 34 weeks of gestation, with symptoms of preterm labour were recruited. The control group consisted of healthy pregnant women of similar gestation age. The maternal serum vitamin D and calcium levels were measured and all women were followed up until delivery. The patients’ demographics data and their respective perinatal outcomes were collected and analysed. A total of 161 women were recruited for the study. The prevalence of vitamin D deficiency, insufficiency, and sufficiency were 41.3%, 50.9%, and 6.8% respectively (p < 0.001). The mean maternal serum vitamin D for control, threatened preterm labour, and preterm birth group were 26.5 ± 3.8 ng/mL, 17.9 ± 8.0 ng/mL, and 13.5 ± 6.98 ng/mL respectively (p < 0.001). There were no significant differences in the means of serum vitamin D among women with or without antenatal complications, such as gestational diabetes, gestational hypertension or preeclampsia. Vitamin D level was positively correlated with gestational age at delivery (p < 0.001) and newborn weight (p < 0.001). Vitamin D deficiency is associated with increased risk of spontaneous preterm labour, although there is no strong evidence of correlation with other antenatal complications. In view of high prevalence of vitamin D deficiency and insufficiency in this population, routine supplement of vitamin D maybe beneficial in reduction of premature births.
Vitamin D is a fat-soluble secosteroid which plays a major role in bone metabolism and mineral homeostasis. Vitamin D is unique such that it functions as a prohormone and involves in a variety of metabolic processes. Under adequate sun exposure, the human body is able to synthesize vitamin D and its deficiency is linked to a spectrum diseases including autoimmunity, insulin resistance, cardiovascular disease, and malignancies [1, 2].
Vitamin D deficiency or insufficiency has been associated with various adverse pregnancy outcomes including gestational diabetes mellitus (GDM), preeclampsia, and small for gestational age (SGA) [3]. Vitamin D deficiency is also linked to an increased rate of primary caesarean section [4].
According to UNICEF report in 2016, preterm birth contributed 34.8% of neonatal deaths worldwide [5], while in Malaysia, prematurity and low birth weight contributed to around 19.4% of perinatal mortality [6]. The etiology of preterm labour is complex and multifactorial. Uterine overdistension, abnormal activation of fetal endocrine pathways, uterine infection, and inflammation were among the contributory factors [7].
In spontaneous preterm labour, there is a switch of the myometrial quiescence to the coordinated contractility prior to fetal maturation. Myometrial contractility depends on vitamin D-regulated calcium release within the muscle cell. Vitamin D therefore has a role in reducing reduce the risk of spontaneous preterm birth by maintaining myometrial quiescence [8]. This study was aimed to determine the serum level of vitamin D in pregnant women complicated with spontaneous preterm birth. The present authors hoped that their findings would justify the role of vitamin D supplementation as a simple and inexpensive method to reduce the risk of preterm delivery in this population.
This prospective observational study was conducted in a tertiary hospital between August 2012 and June 2013. Prior ethics approval was obtained from the National University of Malaysia Ethics and Research Board (Approval Code: FF-356-2012).
| Demographics | Controls (n=75) | Women with symptoms of preterm labour | ||
|---|---|---|---|---|
| Delivered |
Delivered |
p-value | ||
| Age, years; mean (SD) | 29.59 (4.94) | 30.33 (4.62) | 30.18 (4.60) | 0.09 |
| Parity, n (%) | ||||
| Nulliparous | 28 (37.3) | 15 (41.7) | 18 (36) | 0.57 |
| Multiparous | 47 (62.7) | 21 (58.3) | 32 (64) | |
| BMI, kg/m2; mean (SD) | 25.73 (4.32) | 27.22 (4.57) | 24.51 (4.35) | 0.10 |
The inclusion criteria were women with singleton pregnancy between 26 to 34 weeks, presented with regular uterine contractions (at least one contraction within ten minutes, lasting for at least 25 seconds, documented by manual palpation or tocogram), with or without cervical effacement and dilatation. The exclusion criteria were multiple pregnancy, congenital anomalies, and iatrogenic preterm labour following diagnosis such as of preeclampsia or intrauterine growth restriction. The control group consisted of healthy uncomplicated pregnancies of similar gestation age.
Women who fulfilled the inclusion criteria were counselled and consented on admission. Blood was taken for serum vitamin D and calcium level. The 25(OH) D3 in serum was quantitatively determined using an immunoassay analyser and the concentration was expressed in ng/mL. Each value was categorized as the following; deficient (< 20 ng/mL), insufficient (20-30 ng/mL), and adequate (> 30 ng/mL). The corrected serum calcium level was calculated by this formula: corrected calcium (mmol/L) = measured total calcium (mmol/L) + 0.02 (40 - serum albumin [g/L]). The value of 40 represented the average albumin level.
Women with suspected preterm labour were managed as per hospital protocol. Intramuscular dexamethasone to promote fetal lung maturity and treatment with tocolytic agent was administered, if indicated. Those without preterm births were followed up fortnightly until delivery. The women in the control group were recruited among those who attended antenatal clinic for routine appointment and their progress was followed until delivery. Preterm birth before 37 weeks in the control group, were excluded.
The patients’ demographic data, antenatal complications (such as hypertensive disorder and gestational diabetes), gestation at birth, and mode of delivery were documented. Neonates’ data such as cord pH, birthweight, head circumference, and length were also recorded.
All statistical analysis was performed using SPSS version 22.0. Categorical variables were presented as frequencies and percentages, while quantitative variables were presented as mean ± standard deviation (SD). Chi-square test and Fisher-exact test for categorical variables and independent t-test for continuous variables were used when appropriate. Pearson correlation was used to investigate correlation between two variables. In all tests, the level of significance was 0.05.
A total of 161 women were recruited. Eighty-six women had symptoms of preterm labour and 50 of them delivered prematurely. Seventy-five women were included as controls, as shown in Figure 1.
The demographics data for the present women are shown in Table 1. There was no significant difference in age, parity, and BMI between women with symptoms of preterm labour and the controls. The mean gestations at delivery for control, threatened preterm, and preterm group were 38, 37, and 32 weeks, respectively (p = 0.003). Majority of the patients delivered vaginally. There was a significant difference in mean for birth weight amongst the three groups (p < 0.001).
The mean maternal serum vitamin D concentration was 20.5 ng/mL (SD 8.3). The prevalence of vitamin D deficiency and insufficiency amongst our subjects were 42.3% and 50.9%, respectively, while only 6.8% of the present women had adequate vitamin D levels (p < 0.001). The mean for serum vitamin D level for control group was 26.5 ng/mL (SD 3.8), the preterm group was 13.5 ng/mL (SD 6.9), while those with threatened preterm labour was 17.9 ng/mL (SD 8.0). There was no significant difference in the corrected calcium level between all three groups. None of the patient who delivered preterm had adequate vitamin D level while 12.0% of women in the control group had sufficient vitamin D. Three quarters of women in both threatened preterm and preterm delivery groups were vitamin D deficient in comparison to only 5.3% of women in the control group (p < 0.001).
There were no significant differences between all three groups in term of prevalence of GDM, pregnancy-induced hypertension (PIH) and preeclampsia (PE), as shown in Table 2. There were 76 cases of GDM among 161 studied participants. The mean for serum vitamin D level for subjects with GDM was 19.3 ng/mL (SD 8.0) and without GDM was 21.6 ng/mL (SD 8.2), p = 0.10. PIH was diagnosed in 57 subjects. The mean for serum vitamin D level for subjects with PIH was 20.9 ng/mL (SD 7.2) and without PIH was 20.3 ng/mL (SD 8.9), p = 0.08. There were 26 cases of pre-eclampsia. The mean for serum vitamin D level for subjects with PE was 20.4 ng/mL (SD 6.9) and without PE it was 20.5 ng/mL (SD 8.6), p = 0.09. No significant differences were noted in the level of maternal serum vitamin D between the women who developed GDM, PIH and preeclampsia, and those without the antenatal complications.
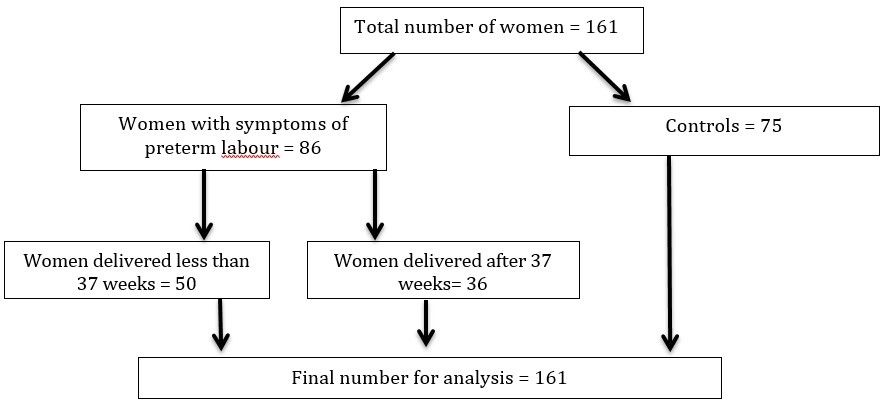 Figure 1.
Figure 1.— Patients’ recruitment.
There were significant differences in mean birthweight amongst the three groups as shown in Table 2. The mean arterial cord pH was similar across the groups. The scatterplot diagram of vitamin D against gestational age at delivery is shown in Figure 2. The Spearman’s correlation test yielded a significant positive correlation between vitamin D and gestation at birth with correlation coefficient of 0.561 (p < 0.001).
 Figure 2.
Figure 2.— Scatterplot of serum Vitamin D level and gestational age at delivery.
Figure 3 represents the scatterplot diagram of maternal vitamin D level against new-born weight. Using the Spearman’s correlation test, the authors found a moderate positive correlation between vitamin D levels and new-born weight with correlation coefficient of 0.512 (p < 0.001).
 Figure 3.
Figure 3.— Scatteplot of vitamin D against birthweight.
| Clinical outcomes | Controls (n=75) | Women with symptoms of preterm labour | p-value | |
|---|---|---|---|---|
| Delivered after 37 weeks (n= 36) | Delivered preterm (< 37 weeks) (n=50) | |||
| Vitamin D ng/mL, mean (SD) | 26.5 (3.8) | 17.9 (8.0) | 13.5 (6.9) | < 0.001 |
| Corrected Calcium level, mean (SD) | 2.24 (0.2) | 2.25 (1.1). | 2.20 (0.2) | 0.17 |
| Vitamin D levels, n (%) | ||||
| Adequate | 9 (12.0) | 2 (5.6) | 0 (0) | p < 0.001 |
| Insufficient | 62 (82.7) | 7 (19.4) | 13 (26.0) | |
| Deficient | 4 (5.3) | 27 (75.0) | 37 (74.0) | |
| Antenatal complications n (%) | ||||
| GDM | 29 (39) | 22 (61) | 25 (50) | 0.08 |
| Gestational HTN | 31 (41) | 18 (50) | 8 (16) | |
| Pre-eclampsia | 13 (17) | 6 (17) | 7 (14) | |
| Gestational age at delivery, weeks; median (SD) | 38 (1.46) | 37 (1.46) | 32 (1.22) | 0.003 |
| Mode of delivery, n (%) | ||||
| Vaginal delivery | 58 (77) | 26 (72) | 39 (100) | 0.04 |
| LSCS | 17 (23) | 10 (28) | 0(0) | |
| Newborn weight, kg; mean (SD) | 3.03 (0.44) | 2.69 (0.47) | 1.78 (0.36) | < 0.001 |
| Cord pH; mean (SD) | 7.29 (0.10) | 7.28 (0.10) | 7.30 (0.10) | 0.98 |
Vitamin D status is influenced by factors which control its production in the skin, such as skin pigmentation, latitude, seasons, and dress code [9]. The prevalence of vitamin D deficiency in pregnancy in light-skinned and dark-skinned or veiled populations had been reported in ranges of 5-20% and 30-70%, respectively [3]. A recent study from Thailand found the prevalence of vitamin D deficiency in women with preterm labour was 48.3%, which was similar to the present cohort. Bhupornvivat et al. however concluded that serum vitamin D level was not predictive of premature labour among Thai women [10]. A meta-analysis of ten observational studies involving 10,098 participants showed that maternal vitamin D deficiency (< 20 ng/mL) was significantly associated with increased risk of preterm birth (OR 1.29, 95% confidence intervals 1.161.45) [11]. The present study found that vitamin D deficiency was strongly associated with preterm birth and there was a positive correlation between vitamin D and gestational age. Shibata et al. demonstrated that hypovitaminosis D is associated with threatened preterm labour [12]. The Japanese study had a lower mean vitamin D level in its threatened preterm labour group in comparison to the present [11.2 ng/ml (SD 3.2) vs. 17.9 ng/mL (SD 8.0)].
Maternal vitamin D deficiency has been associated with increased risk of preeclampsia [13-15]. A systematic review by Wei et al. involving 24 studies found that women with hypovitaminosis D of less than 50 nmol/L were twice more likely to develop preeclampsia (OR 2.09, 95% confidence intervals 1.50-2.90) [16]. Serum concentrations of 1, 25(OH)2D were significantly lower in pre-eclamptic women compared to normal controls [17]. The present study did not find any difference in serum vitamin D level among women with or without preeclampsia. A study by Wetta et al. also found no association between mid-trimester vitamin D level and preeclampsia [18].
There were several studies in the literature which supported the link between vitamin D deficiency and gestational diabetes [19-22]. Zhang et al. found that the maternal plasma 25-[OH]D concentrations at 16-week gestation were 20% lower amongst women who subsequently developed GDM [19]. A recent study by Burris et al. demonstrated that there was an inverse correlation between 25(OH) D levels and blood glucose measurement after a one-hour, 50 gram glucose load [22]. A study by Al-Shaikh et al. in 1, 000 pregnant women from Saudi found no association between vitamin D deficiency and GDM, however they discovered a significant negative correlation between serum 25(OH)D levels and fasting blood glucose among women above 35 years-old [23]. A study by Ramos-Lopez et al. suggested that the single nucleotide polymorphism in the CYP27B1 promoter region was responsible to modulate the 25(OH)D3 serum levels in GDM patients [24]. The present study did not find any significant difference in the mean vitamin D level between women with GDM and those who were normoglycemic. However this could be related to the design of the present study which was not geared to significantly identify these differences.
The present study found that there was a significant positive correlation demonstrated between serum vitamin D levels and new-born weight (p < 0.001). Mannion et al. demonstrated that vitamin D intake in pregnancy is independently associated with infant birthweight, whereby each additional microgram of vitamin D was linked to an increase of 11 grams in birth weight [25]. Two large studies, from the Netherlands and Australia found that women with vitamin D deficiency (using thresholds of < 25 and 29 nmol/L) were noted to have infants with significantly lower birthweights [26, 27]. A meta-analysis by Thorne-Lyman et al. revealed that vitamin D supplementation was associated with a significant 60% risk reduction of low birthweight infants [28] However, there were also studies reporting a lack of significant link between maternal vitamin D status and low birthweight [29-31]. Any relationship between maternal vitamin D status and birth weight may be obscured by multiple confounding factors, including preterm delivery, maternal diabetes or hypertensive disorder, and weight gain during pregnancy.
The present authors believe that this study was the first to assess the link between hypovitaminosis D and preterm labour among pregnant Malaysian Women. They have demonstrated a significant positive correlation between vitamin D and gestation, as well as infant birthweight. The combined prevalence of vitamin D deficiency and insufficiency in the present cohort was 93.2% which was alarmingly high. A cross-sectional study on urban pregnant women in Malaysia discovered that the prevalence of hypovitaminosis D in first trimester was around 90.4% [32]. A recent prospective observational study based in the present centre, found that the prevalence of vitamin D deficiency and insufficiency among antenatal women at term were 71.7% and 21.0%, respectively [33]. Therefore routine vitamin D supplementation for antenatal women is justified in the present population in order to reduce risk of preterm birth and other potential pregnancy complications such as preeclampsia and gestational diabetes. However, further research with serial assessment of serum vitamin D level during pregnancy, taking into account confounders such as infection, is necessary to establish a strong causal relationship between hypovitaminosis D and adverse pregnancy outcomes, as well as determining the optimal supplement dosage for this population.
This study was supported by the Universiti Kebangsaan Malaysia Medical Centre Fundamental Research Grant [FF-356-2012].
