Aim: The aim of this study is to develop and optimize artificial neural network models for accurate prediction of premature ovarian failure (POF), to test these models on data collected prospectively from different centres. Materials and Methods: The study used data from 316 women presenting to six communities governed by a street in Wuhan, Hubei, China. Unbiased randomization was divided into training samples (177 cases), test samples (44 cases), and adherence samples (95 cases). Data from training samples and test samples were used to train the models, which were then tested on independent data from adherence samples. From 35 potential factors, variables were selected by Analytic Hierarchy Process (AHP), and then were used in the ANN model to make the prediction. Results: The predicting accuracy of the train set, validation set, and test set were 98.73%, 94.15%, and 92.15%, respectively, when the generalization ability was verified. Conclusion: This study confirms that artificial neural network can offer a useful approach for developing diagnostic algorithms for POF prediction.
Premature ovarian failure (POF) is a common reproductive endocrine disease. Recent and long-term complications such as fertility loss, osteoporosis, genital atrophy, cardiovascular diseases, and metabolic system diseases are deeply puzzling every patient and family [1]. The factors that affect POF are large and complex, so there are many predictors that need to be considered.
There is a non-linear relationship between different prediction parameters of POF, which leads to the fact that traditional methods cannot accurately describe the correlation between different parameters, and there is a large deviation in prediction results of POF [2]. In this case, the effective prediction mode of POF has become the key research direction of the relevant scholars.
In recent years, with the rapid development of information technology, the prediction models of premature ovarian failure based on multiple linear regression, support vector machine and neural network have emerged [3].
Multiple linear regression is evaluated based on the linear relationship, but it is not good at approximation to the nonlinear problem. Support vector machine is only to solve the small sample, but it is long training time and low operating efficiency when the sample is large [4].
However, the artificial neural network is nonlinear, realtime optimization and intelligent learning ,it has become the main method for predicting premature ovarian failure model. In view of the above problems, this paper proposes a prediction method of premature ovarian failure based on AHP method and BP Neural Network (BPNN) method.
All predictors of premature ovarian failure can be analysed by the AHP and the weights of the influence factors can be calculated. According to the weight, select the higher impact predictors of premature ovarian failure.
The index is considered as the input parameter of the BPNN. After training the neural network model based on clinical data, we can evaluate the accuracy of premature ovarian failure prediction. The experimental results demonstrate the highly accurate evaluation performance of the proposed method.
There were 316 women who met the inclusion criteria, they were collected in six communities governed by a street in Wuhan. Inclusion criteria: (1) the age range was from 35 to 40 years, (2) in the past three months, there was no history of hormone medicine treatment, or history of pregnancy, abortion and lactation, (3) there was as complete uterus and at least one ovary, and no abnormalities were found by B-mode ultrasound, (4) living in six communities for more than one year, (5) there was a clear and spontaneous end of the menstrual period, (6) they had good compliance and were willing to participate in this research. Exclusion criteria: (1) Severe complications, such as malignant tumor, renal failure, etc, (2) endocrine diseases such as polycystic ovary syndrome (PCOS), diabetes, thyroid, and breast.
All the participants signed the informed consent. They all completed the relevant questionnaire survey (a type behavior, history of mumps, history of gynecologic surgery, history of using ovulation drugs, and history of marriage and childbearing). They required an endocrinological examination and ultrasound examination on the third day of menstruation.
From May 2012 to June 2017, the subjects were visited once every four months and followed up until the age of 40. The follow-up was used to identify whether the participants had POF, including menstrual status, female hormones, special medication, and gynecologic surgery, etc. Exclusion criteria: during the follow-up period, the treatment of hormone and psychotropic medicine, or pregnancy, abortion and lactation were performed. At last, two participants underwent hysterectomy, two participants had sex hormone therapy, and 21 participants lost to follow-up. All participants above were eliminated. A total of 316 participants were included in the study.
The first step to build a prediction model for POF is to establish a corresponding prediction parameter set [5]. The prediction of POF is influenced by many factors, such as risk factors, biochemistry, and ultrasound. In this paper, through systematic analysis and expert reviews, referring to the related literature and research, the prediction criteria include risk factor, biochemical index, B ultrasonic index, and Test index are presented, and each evaluation criterion contains multiple sub evaluation indexes. The analytic hierarchy process is used to establish the prediction index system of POF as shown in Figure 1. The relative importance of calculating the same level prediction index is calculated and the comprehensive weight is obtained by AHP. The key step of the AHP is to construct the index judgment matrix. In order to reduce the influence of subjective factors, compared with each other among the predictors of POF and construct judgment matrix A. Matrix A elements in value evaluation index for POF prediction of results is of relative importance, including clinicians’ and experts’ scoring in order to determine and judge matrix elements in the evaluation standards (Table 1). The element value in matrix A indicates the relative importance of predictive indicators of POF. This paper uses both clinician and expert to allocate scores. The criterion of the assignment of elements in matrix A is shown in Table 1.
 Figure 1.
Figure 1.— Neural network prediction model.
| Assignment(Wi/Wj) | Explanation |
|---|---|
| 1 | The two indicators have the same importance |
| 3 | Vi index is slightly more important than Vj |
| 5 | Vi index is obviously more important than Vj |
| 7 | Vi index is more important than Vj |
| 9 | Vi index is more important than Vj |
According to the prediction index matrix, the weights W can be obtained by AW = λmaxW. Then the normalization operation is carried out to calculate the relative importance weights of the upper levels. Finally, a consistency test for this matrix is implemented. Then to test the consistency of the judgment matrix from the high to the lower level. Finally, the forecast index is sorted according to the weight.
The authors sought to exploit the sophisticated pattern recognition capabilities of artificial neural networks for analysis of clinical data from patients presenting with POF. The artificial neural networks used in this study were BP neural network that has been used extensively in medical pattern classification tasks. BPNN consists of the input layer, a number of hidden layers, and the output layer, which is one the most popular networks. The BP algorithm is a supervised learning algorithm. The error back propagation algorithm of BP neural network is a typical supervised learning algorithm. That is, the weight and the threshold of each layer can be adjusted through the forward propagation and back propagation error; the weight adjustment process is the BP neural network learning and training process. The process of reducing the output error is a cycle of reciprocation until it reaches the termination condition.
The topology structure of the neural network is composed of the number of network layers, the number of nodes in each layer, and the connection mode between the nodes [6]. The BP neural network can have one or more hidden layers. The three-layer BP neural network can complete any mapping of n-dimension to m-dimension. That is, an implicit layer has completely been able to simulate any nonlinear relationship. The three-layer neural network is created through the prediction index system of POF based on the analytic hierarchy process as shown in Figure 2. Seven higher weighted prediction indexes in Table 2 are used as input to neural networks, and the three layers’ construction is adopted for the proposed BPNN method. The input nodes is the seven predictors in the analytic hierarchy process and the output node is set as one. The number of hidden layer nodes is determined by empirical formula and preliminary experiment, and is finally set to six. The process of predicting POF is as follows:
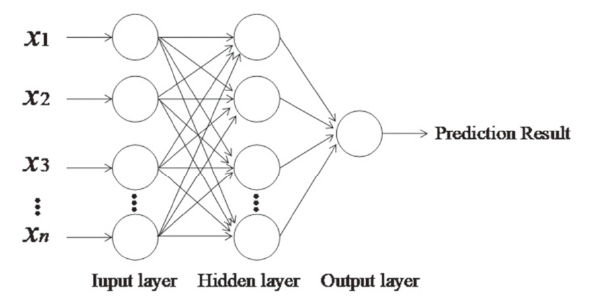 Figure 2.
Figure 2.— Neural network prediction model.
| Factors | Weights |
|---|---|
| Type A behavior | 0.4206* |
| History of mumps | 0.1205 |
| History of Gynecological surgery | 0.4185* |
| History of the use of ovulatory drugs | 0.2053 |
| Obstetrical history | 0.1854 |
| Follicle stimulating hormone | 0.3453* |
| Follicular stimulating hormone / luteinizing hormone | 0.2293 |
| Anti-Müllerian hormone | 0.5448* |
| Inhibin B | 0.4878* |
| Antral follicle count | 0.4556* |
| Peak systolic velocity | 0.2843 |
| Resistance index | 0.2214 |
| Clomiphene stimulation test | 0.3665* |
| Exogenous FSH stimulation test | 0.1604 |
*Top seven high weight prediction indicators.
Step 1: The input layer is set to Xk =(x1, x2, ..., xn), where xi represents the prediction related index of the input layer. In addition, the ownership value and the neuron threshold are assigned a random number of the distribution over (0, 1). Step 2: The corresponding output layer is set to Y = y. Step 3: The inputs for each unit of the hidden layer are: where, wij is the connection weight of the input layer to the hidden layer, θj is the threshold of the hidden layer unit, and p is the number of hidden layer units. The model activation function uses the Sigmoid function, that is f (x) = 1 / (1+e -x). Then the output of the hidden layer unit is: Step 4: the input to the output layer unit is: the output of the output layer unit is:
Where, vjt is the connection weight from the hidden layer to the output layer; γt is the threshold of the output layer unit.
Steps 1 through 4 are the forward calculation propagation of the model. In the process of error back propagation, the BP neural network should be trained to adjust the threshold γt and the connection weights wij and vjt, so as to continuously
reduce the error to the required accuracy range. Where equation (4) is the final constructed prediction model.
Step 5: Weight correction.
Recursively from the output layer to the hidden layer, the formula is:
Where, wij (t) is the connection weight from neuron i (input layer or hidden layer neuron) to upper layer neuron j (hidden layer or output layer neuron) at time T.
yi is the actual output of neuron i at time T. η is the step adjustment factor, 0 < η < 1. α is the smoothing factor, 0 < α < 1. δj is a value related to the deviation. For the hidden node,
where xj is the actual output value of the hidden node j. for the output node,
Where tj is the expected value of the output. The above steps are cycled until the weight is stable. At this time, the error of the actual output value of the ovarian premature aging predictor index and the expected output value of the output layer becomes sufficiently small, and the ovarian premature aging prediction result is outputted.
In order to test the performance of the prediction model of AHP-BPNN POF, the simulation experiment was carried out on the clinical data platform. The experimental data came from the Wuhan clinical medical scientific research project (WX15D15) “Research on prediction model of premature ovarian failure based on artificial neural network”, and a total of 316 data were collected. Each data included 14 evaluation indicators, and some of the data are shown in Table 3.
| Number | X1 | X2 | X3 | X4 | … | X14 | Y |
|---|---|---|---|---|---|---|---|
| 1 | 0.02 | 14.49 | 0 | T | … | 78.71 | 0.87 |
| 2 | 0.36 | 19.63 | 0 | T | … | 75.95 | 0.75 |
| 3 | 0.33 | 14.05 | 0 | T | … | 56.82 | 0.64 |
| 4 | 0.13 | 13.38 | 0 | F | … | 91.57 | 0.60 |
| 5 | 2.90 | 89.40 | 3 | T | … | 15.40 | 0.50 |
| 6 | 2.52 | 69.71 | 3 | F | … | 19.60 | 0.32 |
| 7 | 3.90 | 88.30 | 6 | T | … | 6.69 | 0.86 |
| 8 | 5.83 | 106.39 | 10 | F | … | 8.29 | 0.84 |
| 9 | 6.32 | 105.29 | 9 | F | … | 8.52 | 0.16 |
| … | … | … | … | … | … | … | … |
| 316 | 8.16 | 92.93 | 9 | F | … | 8.58 | 0.83 |
Too large or too small the sample data value will increase the computational complexity and the length of training. To this end, it is normalized and zoomed to a closed interval [0,1], which is shown as follows:
$X_{i}^{’}=\frac{X_{i}-X_{im in}}{X_{imax}-X_{i min}}$
where represents Xi the ith index. Xi min and Ximax represent the minimum and maximum value of the ith index respectively; X i′ represents the normalized value.
The AHP is adopted to obtain the weight of the predictive index. Finally seven indicators are achieved, such as AMH, INHB, AFC, A type behavior, surgery, clomiphene provocation test, and FSH. Then, the data of the sven indexes are processed to achieve a new set of data. The data is divided into two parts, in which 263 data are selected as the training sample set, and the remaining 53 data are used as the test sample set. The training samples are regarded as the input of the BPNN for training. The specific training process of the BP neural network is shown in Figure 3. The test set is assessed by using to establish optimal prediction model of POF, and actual output and model output results are shown in Figure 4. The correlation coefficient between the two types of output is 0.9450, and the prediction accuracy is 94.73%. The results showed that the effectiveness and the feasibility of the proposed method for predicting POF. In order to further detect the generalization ability of AHP-BPNN, 316 data are divided into training set, validation set, and test set.
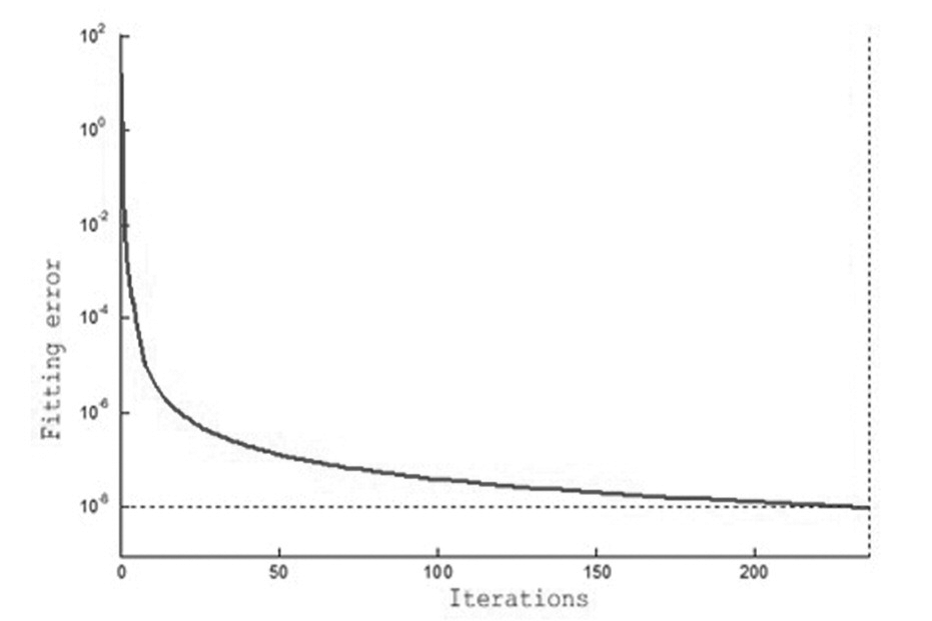 Figure 3.
Figure 3.— The learning process of BP neural network.
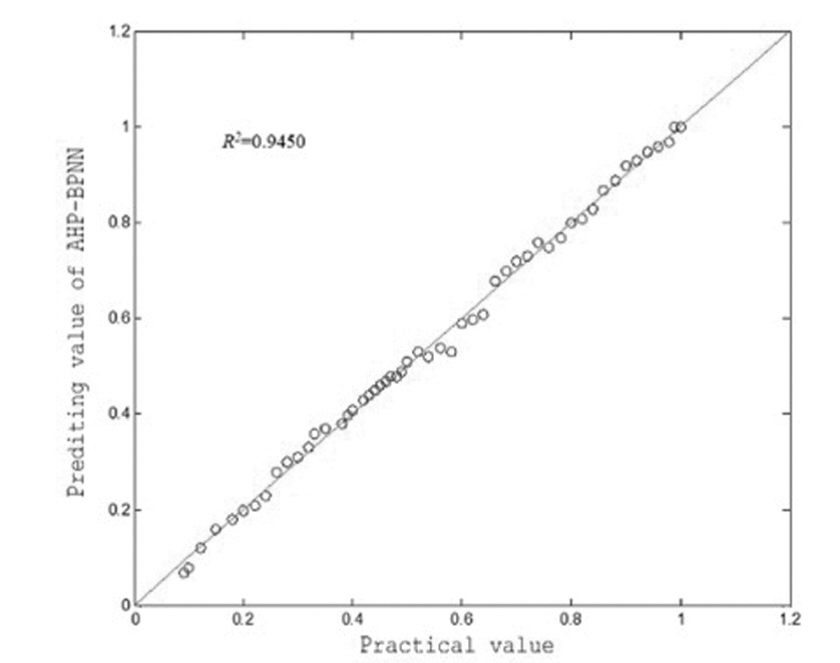 Figure 4.
Figure 4.— AHP-BPNN real output and model output correlation change curve.
The number of samples is 210, 84, and 22, respectively. First, the training set is input into the BPNN for learning. Then, the validation set is used to demonstrate validity of the proposed model. Finally, the optimal model is selected to examine the test sets. The predicting accuracy of the training set, the validation set. and the test set are 98.73%, 94.15%, and 92.15% respectively. The high predicting accuracy of the test set indicates that the generalization ability of the AHP-BPNN model is good, and it can avoid the problem of overfitting.
In this study, a screening by AHP important predictor of POF is AMH, INHB, AFC, A-type behavior, history of gynecological surgery, clomiphene citrate challenge test, and FSH. Both AMH and INHB are members of the transforming growth factor β superfamily. The former level has nothing to do with the menstrual cycle and is secreted by the antral follicles and granule cells of the sinusoidal follicles. It is positively correlated with the number of retrieved eggs and ovarian reactivity [7].
The latter level began to increase from the follicular phase, reached the peak of ovulation period, the luteal phase gradually decreased, secreted by granulosa cells, feedback inhibition of pituitary secretion of FSH, with a direct reflection of ovarian reserve function.
The antral follicle number refers to the sum of the number of all sinusoidal follicles (2-10 mm in diameter) in both ovaries measured by ultrasound. Sinusoidal follicles are highly sensitive and responsive to FSH, and are highly correlated with INH and AMH, so this indicator is often used clinically to reflect the remaining follicular pool of the ovary. In the process of ovarian dysfunction, the decrease in the number of primordial follicles is consistent with the decrease in the number of FSH-sensitive sinusoidal follicles, so this indicator can reflect the state of ovarian reserve [8]. Chen et al. proposed that the critical value of AFC for predicting ovarian dysfunction: AFC ≤ 7 (any age) or AFC ≤ 10 (38 years or older) [9]. It is noteworthy that although AFC gradually decreases with age, and is closely related to the age of sterilization and menopause, only a small amount of AFC can be used to make effective clinical predictions [10]. Project monitoring is more subjective, so AFC has certain limitations as a predictor [11]. People with Type A behavior are subject to fluctuating emotions, and even if they rest, they cannot relax. They lack patience, have a strong sense of time, and do things quickly. Depression, disharmony with family members, and type A behavioral response pattern interfere with the hypothalamic-pituitaryovarian axis, resulting in negative conditioned reflexes and further abnormal secretion of hypothalamic FSH, LH, and estrogen (E2), menstrual cycle changes, and eventually develop into amenorrhea. Many studies at home and abroad show that different gynecological procedures and paths, intraoperative resection range, resection area, and different hemostasis modes all have different effects on ovarian function, patient’s psychology, and postoperative quality of life [12].
In recent years, because laparoscopic techniques have the advantages of no laparotomy, less trauma, quicker recovery, and less postoperative pain, the use of laparoscopic techniques has gradually increased in ovarian surgery [13]. Electrosurgical instruments are applied during the laparoscopic surgery. Electric heating may damage ovarian tissue or affect ovarian blood flow, thereby affecting ovarian function [14]. Therefore, gynecological surgery, especially laparoscopic surgery, requires a correct understanding of the use of electrosurgical equipment, strict control of surgical methods, and surgical indications, selection of the appropriate way to stop bleeding, thereby reducing the impact on ovarian reserve function. It not only achieves a minimally invasive impact on the surface, but also achieves a minimally invasive affect on ovarian function [15]. Clomiphene, as an estrogen receptor antagonist, can directly compete with the estrogen receptor on the hypothalamic pituitary, and prevent the inhibitory effect of estrogen on FSH. In the menstrual cycle of 5-9 days, a daily dose of 100 mg clomiphene, and monitoring the change of FSH level. After withdrawal, when the E2 and INHB secreted by growth follicles could not inhibit the increase of FSH, that is, FSH > 20I U/L or two standard values above the baseline value. It indicated that the ovarian reserve function decreased, which was the occult phase of POF. Researchers believe that the best indicator for predicting ovarian reserve status is CCCT [16].
Using the above single indicators to predict POF failure, their accuracy is limited. With the development of predictors of POF, a complex approach to multiple factors has been developed, and a prediction method for POF based on AHP-BPNN has been proposed in this paper and been tested through clinical trials. The results show that AHP-BPNN uses AHP to filter the importance indicators, simplifies the neural network model structure, and greatly reduces the model computation time. At the same time, the BP neural network with non-linear approximation ability can predict complex POF.
The prediction accuracy of POF and the operating efficiency of the predictive system are more scientific and accurate, and have good application prospects in clinical practice.
The work described in this paper was supported by the Health and Family Planning Commission of Hubei, China, (Grant No. WJ2018H0102) and the Fourth Batch of Wuhan Middle-Young Medical Talents Project in Hubei, China. These financial contributions are gratefully acknowledged.
