Angiogenesis is important to folliculogenesis and oocyte maturation. Vascular endothelial growth factor (VEGF) in follicular fluid (FF) induces folliculogenesis. Angiotensin II is involved in angiogenesis through angiotensin II receptors. To elucidate the mechanisms of oocyte maturation disorders in endometriotic patients, the authors investigated the concentration of VEGF, angiotensin II, and soluble receptor of VEGF (sFlt-1) in FF and the expression of angiotensin II receptors mRNA in granulosa-lutein (GL) cells in endometriosis patients with controlled ovarian stimulation. FF samples were obtained from 56 IVF cycles (control = 47, endometriosis = 9). GL cells were obtained from 15 IVF cycles (control = 10, endometriosis = 5). The authors examined the concentration of VEGF, sFlt-1, and angiotensin II in FF by ELISA, and the expression of angiotensin II receptors mRNA by quantitative RT-PCR. Informed consents were obtained from all patients and IRB approval was obtained. The expression levels of angiotensin II type 1 and type 2 receptor mRNA in endometriosis was significantly lower than the control. The number of immature oocytes was significantly higher in endometriosis than in control. Lower expression of angiotensin II receptors may affect folliculogenesis and oocyte maturation.
Endometriosis is an enigmatic disease characterized by the ectopic presence of endometrial glands and stroma and is associated with pelvic pain, adhesion formation, and infertility. Endometriosis affects 5% to 10% of women of reproductive age [1], and among women with infertility, 30% to 50% are diagnosed with endometriosis by laparoscopy [2, 3]. Although artificial reproductive technology has been developed, pregnancy rates were lower in endometriosis patients [4]. Gupta et al. reported that in in vitro fertilization (IVF) cycles, the number of follicles in patients with endometrioma was significantly lower than the number in the patients without endometrioma [5]. Demirol et al. reported that in endometriosis patients, the total recombinant follicular stimulation hormone (FSH) dose was significantly higher and the number of mature oocytes was significantly lower in patients who underwent ovarian surgery before intracytoplasmic sperm injection (ICSI) cycles than the patients without ovarian surgery [6]. These reports suggested that endometrioma and cystectomy may affect folliculogenesis and oocyte maturation in endometriosis patients.
Angiogenesis is associated with follicular development and oocyte maturation [7]. Vascular endothelial growth factor A, usually referred to as VEGF, is a mitogen related to increased angiogenesis [8]. Several reports have described the presence of VEGF in follicular fluid (FF) [7, 9]. Soluble fms-like tyrosine kinase-1 (sFlt-1) is a soluble receptor of VEGF, and acts as an inhibitor of VEGF bioactivity, and it has been found in FF [10].
Angiotensin II is a potent stimulator of angiogenesis, and is present in high concentrations in FF [11, 12]. Angiotensin II acts by binding to different receptors: angiotensin type 1 (AT1) or angiotensin type 2 (AT2) receptors [13]. AT1 and AT2 have been identified in human granulosa-lutein (GL) cells [14].
The authors examined the levels of VEGF, angiotensin II, and their receptors in FF and GL cells to elucidate the mechanisms of oocyte maturation disorders in endometriosis patients underwent cystectomy.
The authors studied the concentration of VEGF, sFlt-1, and angiotensin II in FF by enzyme-linked immunosorbent assay (ELISA). FF samples were obtained by 64 IVF cycles at the time of oocyte retrieval performed between 2014 and 2015 in Nihon University Itabashi Hospital. Of the 56 samples of FF, 47 samples were obtained from non-endometriotic patients as a control group (patients’ age range, 28-44 years; mean ± SD., 38.2±4.112), and nine were from the endometriotic group (patients’ age range, 37-42 years; mean ± SD., 39.4±1.810). The authors examined the expression of AT1 receptor mRNA and AT2 receptor mRNA in GL cells by quantitative RT-PCR. GL cells were obtained from 16 IVF cycles performed between 2014 and 2015 in Nihon University Itabashi Hospital. Among 15 GL cells samples, ten were from the non-endometriosis group (patients’ age range, 35-44 years; mean ± SD., 40.1±3.107), and five were from the endometriotic group (patients’ age range, 38-43 years; median ± SD., 39.6±2.074). Endometriosis was diagnosed by surgery performed in Nihon University Itabashi Hospital or other hospitals. Informed consent was obtained from all the patients participating and Institutional Review Board (IRB) approval was obtained.
For controlled ovarian stimulation, the authors used multiple protocols that consisted of gonadotropin, clomifen citrate, aromatase inhibitor, and gonadotropin releasing hormone (GnRH) antagonist. The protocols including GnRH agonist such as the long protocol and the short protocol were excluded because of the pathophysiology of endometriosis. When a follicle reached a diameter of more than 18 mm, a GnRH agonist or human chorionic gonadotropin (hCG) was administered to induce final oocyte maturation. Thirty-five hours after the GnRH agonist or HCG administration, transvaginal oocyte retrieval was performed.
FF samples were collected after identification of the oocyte cumulus complex, centrifuged to remove the cellular components, and stored at -80℃ until analysis. The VEGF, sFlt-1, and angiotensin II concentrations were determined by ELISA. All samples were run in duplicate.
GL cells were collected from FF and purified immediately after egg retrieval. GL cells were lightly centrifuged after removal of the oocyte. The cells were washed in isolation medium twice, and separated from red blood cells using a 50% Percoll gradient. Total RNA was isolated from GL cells using a TRIZOL Reagent. cDNA was synthesized from total RNA using a high capacity cDNA Reverse Transcription Kit. The reaction mix (20 µl) containing 500 ng of the total RNA was incubated at 25℃ for ten minutes, at 37℃ for 120 minutes, and at 85℃ for five seconds for reverse transcriptase inactivation. Quantitative real-time PCR was carried out with cDNA using a 7500 Real Time PCR System. The primer sequence of the AT1 receptor was AGC CAG CGT CAG TTT CAA CC for the forward sequence and ACA AGC ATT GTG CGT CGA AG for the reverse sequence [15]. The primer sequence of the AT2 receptor was AGA ACA GGA TAA CCC GTG ACC for the forward sequence and AGG ATG GCA AAA GGA AGT GC for the reverse sequence [15]. For normalization, the expression of β-actin mRNA was also quantified. Each PCR well received iQ SYBR Green Supermix, and amplification was performed under the following conditions: 95℃ for three minutes, 45 cycles of 95℃ for 15 seconds, and 60℃ (for AT1 receptor) and 59℃ (for AT2 receptor) for one minute. All assays were performed in triplicate.
The qRT-PCR data were analyzed using the comparative CT method, as previously described [15, 16]. The difference in cycle time (ΔCT) was determined as the difference between the number of cycles required for the amplification of β actin. The authors then obtained ΔΔCT by finding the difference between groups. The results were expressed as the fold difference (2-ΔΔCt) [17] compared with the value for the control.
The authors counted the number of oocytes retrieved in cycles obtained GL cells. They compared the frequency of immature oocytes in both groups after the model of a precedent [18].
Mann-Whitney U-test was used to compare the value of VEGF, sFlt-1, angiotensin II, and angiotensin II receptors mRNA between both groups. Fisher’s exact test was used to compare the number of immature oocytes retrieved. Statistical analyses were performed using Graph Pad Prism 6. A p-value < 0.05 was considered significant.
The concentrations of VEGF did not show significant difference between the control and endometriosis groups. The concentrations of angiotensin II did not show significant difference between the control and endometriosis groups. The concentrations of sFlt-1 did not show significant difference between the control and endometriosis groups.
The expression level of AT1 receptor mRNA of the endometriotic group was significantly lower than that of the control group (p = 0.0186) (Figure 1). The expression level of AT2 mRNA of the endometriosis group was significantly lower than that of the control group (p = 0.0193) (Figure 2).
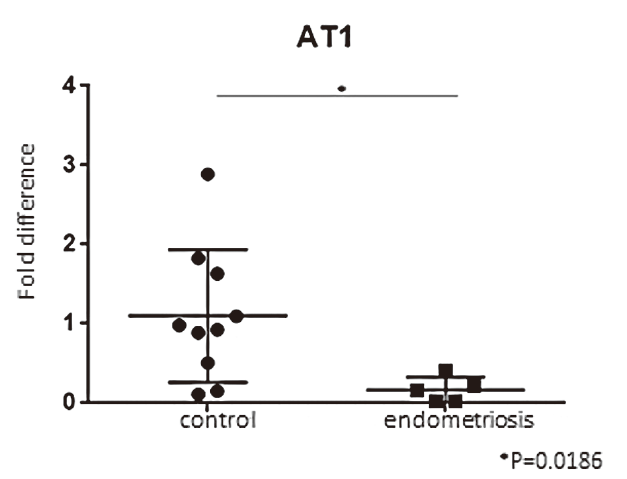 Figure 1.
Figure 1.— This graph shows the expression levels of AT1 receptor mRNA in granulosa-lutein cells by qRT-PCR. The expression level of AT1 receptor mRNA of endometriotic group was significantly lower than that of the control group.
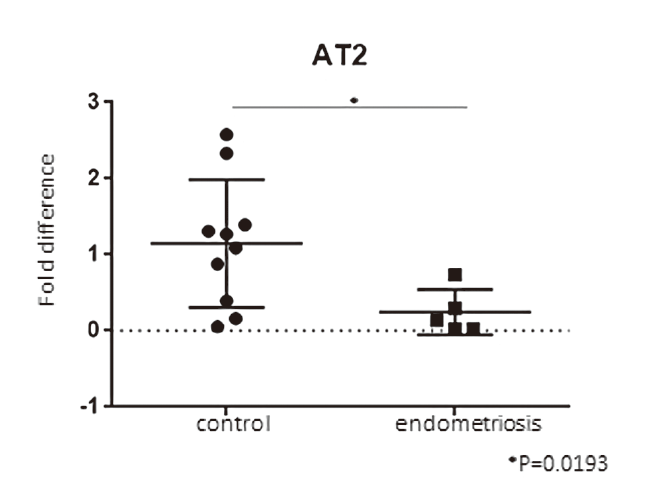 Figure 2.
Figure 2.— This graph shows the expression levels of AT2 receptor mRNA in granulosa-lutein cells by qRT-PCR. The expression level of AT2 receptor mRNA of the endometriotic group was significantly lower than that of the control group.
In the control group, a total of 40 oocytes were obtained including only one immature oocyte. In the endometriotic. group, a total of 13 oocytes were obtained including three immature oocytes. The frequency of immature oocytes was significantly higher in the endometriotic group than in the control group (p = 0.0415) (Table 1).
| MII | Immature oocyte | Total | |
|---|---|---|---|
| Control | 39 | 1 | 40 |
| Endometriosis | 10 | 3 | 13 |
| Total | 49 | 4 | 53 |
Fisher’s exact test; p = 0.0415.
It has been reported that VEGF may play an important role in follicular growth and development [19]. Nishigaki et al. reported that the concentrations of VEGF in FF with diameter ≥ 15 mm were significantly higher than those in FF with a diameter ≤ 14 mm [20]. Monteleone et al. estimated perifollicular vascularity through power Doppler blood flow and graded follicles by percentage of follicular circumference in which blood flow was identified. They reported that the levels of VEGF in FF of high-grade follicles were significantly higher than the levels of low-grade follicles [21]. In natural cycles of IVF treatment, the VEGF concentrations in FF of endometriosis patients were lower than that of non-endometriosis patients [22]. On the other hand, the VEGF concentrations in FF correlated with the total number of gonadotropin [23, 24]. These reports supported the theory that there are no differences between the VEGF concentrations in FF of endometriosis patients and that of controls undergoing ovarian stimulations. SFlt-1 acts as a negative modulator for the bioactivity of VEGF [25]. Gruemmer et al. reported that FF and granulosa cells control VEGF availability by downregulation of the sFlt-1 leading to an increase of bioactive VEGF [10]. In the present study, there were no differences between the VEGF and sFlt-1 concentrations in FF of endometriotic patients and those of the control group; thus, VEGF may not affect folliculogenesis disorder or increase the number of immature oocytes in endometriotic patients who undergo ovarian surgery before ovarian stimulations. In both groups, angiotensin II was present in high concentrations in FF; however, the expression levels of angiotensin II receptors mRNA were significantly lower in the endometriotic group than in the control group. Angiotensin II acts in an antagonistic way by binding to different receptors. The key functions of the AT1 receptor are to promote angiogenesis, increase vascular resistance, and cell growth and development like an anti-apoptotic effect [14, 26]. Conversely, the key functions of the AT2 receptor include vasodilation and inhibition of cell growth [27]. In GL cells, angiotensin II induces apoptosis via AT2 receptors [28]. Changes in granulosa cell cycle kinetics have been reported to possibly be responsible for impaired follicle growth and oocyte maturation in endometriotic patients [29], and there is a proper balance between proliferation and apoptosis in GL cells for obtaining a fully competent oocyte [14]. For endometriotic patients that underwent ovarian surgery, the number of immature oocytes increased and stronger ovarian stimulation was necessary to obtain MII oocytes [6]. The expression of angiotensin II receptors mRNA of endometriosis with ovarian surgery was low; thus, surgery for endometrioma may induce the collapse of the proper balance of GL cell cycles and may affect folliculogenesis and oocyte maturation in endometriotic patients with controlled ovarian stimulation.
