Introduction: Sudden intrauterine death syndrome (SIUDS) and sudden infant death syndrome (SIDS) are often associated in a single pathology, resulting from an unexpected fetal or infant injury. Among the various causes, the action of external toxics, still current in the West, should not be excluded. The present histopathological observations indicate the brainstem nuclei as possible neuronal targets of toxic substances; these cause direct damage to cells, including those at the mitochondrial level, as well as indirect functional impairment. During fetal life in utero, the placenta does not act as a total filter; rather it proves permeable to toxics which are able to penetrate the hematoencephalic barrier which shields the fetus. Clinical tests have yet to be devised which reliably signal impending danger of unexpected fetal or infant injury from external toxics.
The prevention campaigns have decreased without annulling the current incidence of sudden intrauterine unexpected death syndrome (SIUDS) and sudden infant death syndrome (SIDS). The problem of SIUDS and SIDS thus continues to arouse interest, particularly in developed Western countries, given the social impact of these interrelated syndromes [1, 2]. New investigative procedures have enhanced our understanding of this field [1-3]. It is well known that complications in a pregnancy may derive from maternal health factors, such as pre-existing diseases, abnormal habits, and advanced age. Further complications include the situation of the fetus (restricted growth, preterm labor) or placental impairment [4-8]. Recently, external toxic agents present in the soil, either as pesticides, metals or additives, have been known to transmigrate and contaminate agricultural products. Over a three-year period (2011-2013), such agents were detected in parts-per-billion using gas chromatography-mass spectrometry (GC-MS) on real brain matrix. The concentration ranged from a minimum of 0.8 ng/g to a maximum of 34.0 ng/g. The case sample included eight out of 15 SIUDS victims and three out of eight SIDS victims. All victims came from intensively farmed areas of Northeast Italy; the mothers were not active smokers. The toxic agents detected, some of which banned for decades but biopersistent, included: organochlorine compounds, α-chlordane, γ-chlordane, heptachlor, dichlorodiphenyldichloroethylene (DDE), dichlorodiphenyltrichloroethane (DDT), and the two most common organophosphorus pesticides, chlorpyrifos and chlorfenvinphos [9-13]. The pollutants were found individually in six cases of SIUDS, coupled in three cases (α-chlordane plus γ-chlordane in one case of SIUDS, and in one case of SIDS; γ-chlordane plus DDT in one case of SIUDS), and up to three simultaneously in two cases of SIDS (α-chlordane plus γ-chlordane plus heptachlor, chlorpyrifos plus chlorfenvinphos plus DDE). All the fetuses arrived already fixed in formaldehyde from the obstetrics department and, therefore, it was not possible to carry out investigations on their blood or body burdens.
Toxics can enter the mother’s blood externally by various routes, such as direct contact, ingestion or inhalation. They can also be produced by altered maternal metabolisms, passing into the fetal blood via the placenta [14, 15]. It is for this reason that the notional definition of placenta as an absolute safety barrier should be modified into that of a system characterized by selective permeability and differentiated absorption capacity. Its complex structure comprises two epithelial layers, which line the chorionic villi, and two endothelial layers, pertaining to the feeding vessels. By contrast to the single endothelium of the systemic capillaries, this four-membrane structure poses an effective barrier to low-concentration water-soluble substances passing through them. Conversely, high doses of water-soluble compounds can attain appreciable concentrations in the fetal blood. In addition, due to a mechanism of passive diffusion, lipid-soluble chemicals, even in minimal concentrations, are able to pass through the placental barrier [16]. Certain of these lipid-soluble substances have been classified as “endocrine disruptors” (EDs) due to their particular mode of action. They exert selective toxicity on various endocrine systems [9, 11, 17], including autocrine and paracrine, both of which regulate the development of neuronal structures in the fetal brain [13]. Significantly, the chemical structure of certain EDs, e.g. nonylphenols, shows a marked affinity with that of natural hormones, e.g. estradiol [18-20]. EDs and other chemical toxics can cause cell damage through direct injury to cell structures, such as membranes, lysosomes, mitochondria, and nuclei [21-23].
From a pathogenetic and clinical point of view, the present authors feel that attention should be drawn to the fact that pesticides were detected in SIUDS and SIDS victims.Concomitant morphological alterations were detected in nerve structures in the brainstem nuclei, which control many vital vegetative functions, in particular breathing [24]. This function is regulated by various neuronal centers, which together form a “respiratory network”: they chiefly affect the facial/parafacial complex (Figure 1), but other nuclei are also involved, such as the raphe obscurus (Figure 2), the Kölliker-Fuse, the Bötzinger, and the intermediolateral nucleus. The facial/parafacial complex, located in the caudal pons just ventral to facial motor neurons, includes many neurons with a prominent role in rhythmogenic mechanisms and chemoreception [25]. The raphe obscurus nucleus is another chemosensitive center in the medulla oblongata involved in ventilator drive; it contains serotonergic neurons acting as CO2 sensors that are able to maintain pH homeostasis, thus playing a key role in breathing control [26, 27]. In cases of maternal nicotine abuse, significant alterations in SIUDS and SIDS victims were detected in cerebellar areas provided with cholinergic transmission connections functional to respiratory control. These alterations comprised partial or total disruption of the argyrophilic nucleolar organizer region (AgNOR) of the Purkinje cells [28]. Related to the same syndromes, and similarly associated to maternal nicotine abuse, expression of the brain-derived neurotrophic factor (BDNF) was found to be significantly depressed in the granule layers of the cerebellar cortex, chiefly the posterior lobule, a region involved in respiratory control [29]. This implies degeneration in synaptic transmission in the respiratory circuits, with obvious deleterious consequences for survival. Moreover, through an intermediate action, EDs can reduce the level of orexin, a neuropeptide synthesized by neurons in the lateral hypothalamus, thus depressing the respiratory nuclei in the brainstem. The Kölliker-Fuse and coeruleus are particularly affected. These nuclei are directly involved in respiratory dynamics, mainly at the moment of birth [30]. At sub-cellular level, ultrastructural observations of the same brainstem nuclei suggest direct toxicity of the mitochondria. In all likelihood, this develops as a result of the following cascade of events: a primitive mutation in the mitochondria DNA copies leads to impaired respiratory function, compensated by increased mitochondrial biogenesis. This suggests the prospect that we may indeed find related plasma markers [31].
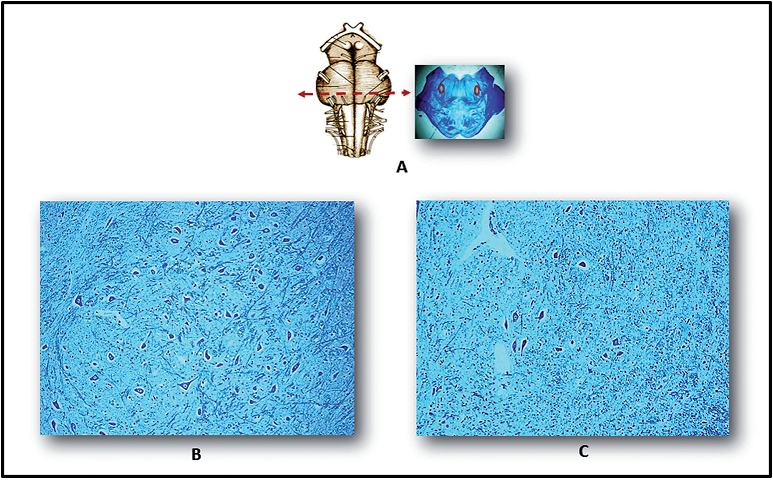 Figure 1.
Figure 1.— Images of the parafacial nucleus in the caudal pons. A) Left: schematic view of ventral brainstem, showing optimal sampling level in nucleus. A) Right: histological section at this level (the circles show bilateral nucleus position in a full-term fetus that died from chorioamnionitis, taken as control). B) Normal cytoarchitecture is seen in a full-term fetus died from chorioamnionitis. C) Hypoplasia of the parafacial nucleus in a case of full-term SIUDS from the present series, comparable for age and body weight with the control [A) Right: Klüver-Barrera staining, magnification ×0.5. B) and C): Klüver-Barrera staining, magnification ×20].
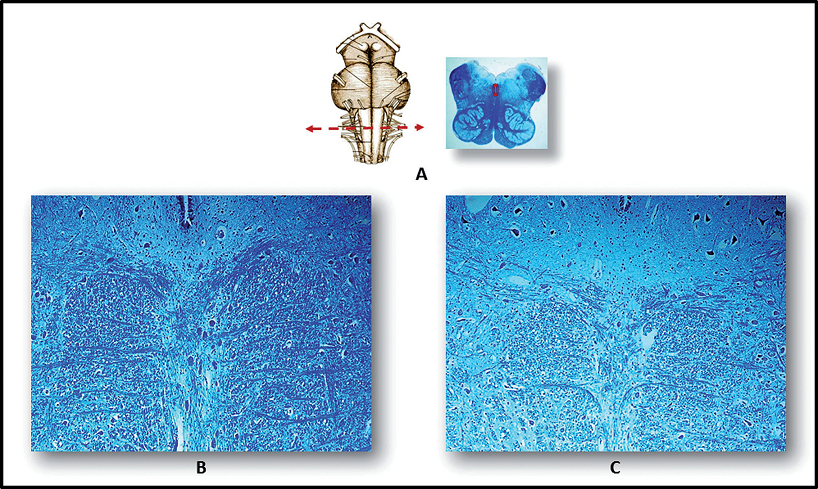 Figure 2.
Figure 2.— Images showing the raphe obscurus nucleus in the medulla oblongata. A) Left: schematic image of the ventral brainstem, showing optimal sampling level in nucleus. A) Right: histological section at this level (the circle indicates the position of the nucleus in a full-term fetus that died from chorioamnionitis, taken as control). B) Normal cytoarchitecture, with serotonergic neurons arranged along the midline, is seen in a full-term fetus that died from chorioamnionitis. C) Hypoplasia of the raphe obscurus nucleus in a case of full-term SIUDS from the present series, comparable for age and body weight with the control [A): Right - Klüver-Barrera staining, magnification ×0.5. B) and C): Klüver-Barrera staining, magnification ×20].
Translating observed results into clinical diagnostics and therapeutics is by no means simple; accordingly, only general prophylaxis and environmental protection lines can be traced. In addition, EDs may act on various factors which regulate fetal brain development, and this further complicates a scenario whose dynamics are as yet not completely understood. All things considered, what the present authors are here proposing is a unified concept of severe and unexpected external aggression which would embrace both SIUDS and SIDS. Nevertheless, a doubt remains regarding damage to other cerebral functions, including the network of respiratory nuclei and about the possibility of recovery. In addition, the dividing line between organic hypoplasia and simple functional immaturity has yet to be clearly defined. As yet, no blood tests or specific markers have been reliably correlated with fetal or infant aggression from external toxics. On the other hand, several neuromorphological studies have demonstrated that altered cellular values (low neuronal nuclear antigen content and high mitochondrial DNA content, respectively) suggest neuronal immaturity and brain-cell damage. Both conditions can be correlated with SIUDS and SIDS. These observations all confirm the fact that fetal brain tissue is singled out and targeted by toxic agents. They address the need for anatomopathological diagnostic procedures [32], indicating that further clinical research is required.
